Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by astrocytes and microglia
- PMID: 19998480
- PMCID: PMC3767435
- DOI: 10.1002/glia.20952
Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by astrocytes and microglia
Abstract
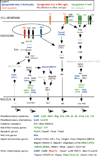
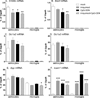
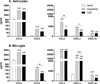
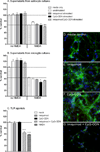
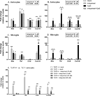
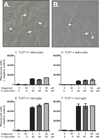
Toll-like receptors 7 (TLR7) and 9 (TLR9) are important mediators of innate immune responses. Both receptors are located in endosomal compartments, recognize nucleic acids, and signal via Myeloid differentiation factor 88 (MyD88). In the current study, we analyzed TLR7 and TLR9 induced activation of astrocytes and microglia, two cell types that contribute to innate immune responses in the CNS. TLR7 and TLR9 agonists induced similar cytokine profiles within each cell type. However, there were notable differences in the cytokine profile between astrocytes and microglia, including the production of the anti-inflammatory cytokine IL-10 and antiapoptotic cytokines G-CSF and IL-9 by microglia but not astrocytes. Costimulation studies demonstrated that the TLR7 agonist, imiquimod, could inhibit TLR9 agonist-induced innate immune responses, in both cell types, in a concentration-dependent manner. Surprisingly, this inhibition was not mediated by TLR7, as deficiency in TLR7 did not alter suppression of the TLR9 agonist-induced responses. The suppression of innate immune responses was also not due to an inhibition of TLR9 agonist uptake. This suggested that imiquimod suppression may be a direct effect, possibly by blocking CpG-ODN binding and/or signaling with TLR9, thus limiting cell activation. An antagonistic relationship was also observed between the two receptors in microglia, with TLR7 deficiency resulting in enhanced cytokine responses to CpG-ODN stimulation. Thus, both TLR7 and its agonist can have inhibitory effects on TLR9-induced cytokine responses in glial cells.
(c) 2009 Wiley-Liss, Inc.
Figures




Similar articles
-
The early induction of suppressor of cytokine signaling 1 and the downregulation of toll-like receptors 7 and 9 induce tolerance in costimulated macrophages.Mol Cells. 2015 Jan 31;38(1):26-32. doi: 10.14348/molcells.2015.2136. Epub 2014 Dec 15. Mol Cells. 2015. PMID: 25518931 Free PMC article.
-
Innate immune responses after stimulation with Toll-like receptor agonists in ex vivo microglial cultures and an in vivo model using mice with reduced microglia.J Neuroinflammation. 2021 Sep 6;18(1):194. doi: 10.1186/s12974-021-02240-w. J Neuroinflammation. 2021. PMID: 34488805 Free PMC article.
-
IL-4 suppresses the responses to TLR7 and TLR9 stimulation and increases the permissiveness to retroviral infection of murine conventional dendritic cells.PLoS One. 2014 Jan 29;9(1):e87668. doi: 10.1371/journal.pone.0087668. eCollection 2014. PLoS One. 2014. PMID: 24489947 Free PMC article.
-
Regulatory molecules required for nucleotide-sensing Toll-like receptors.Immunol Rev. 2009 Jan;227(1):32-43. doi: 10.1111/j.1600-065X.2008.00729.x. Immunol Rev. 2009. PMID: 19120473 Review.
-
Antiinfective applications of toll-like receptor 9 agonists.Proc Am Thorac Soc. 2007 Jul;4(3):289-94. doi: 10.1513/pats.200701-021AW. Proc Am Thorac Soc. 2007. PMID: 17607015 Free PMC article. Review.
Cited by
-
The two-component adjuvant IC31® boosts type i interferon production of human monocyte-derived dendritic cells via ligation of endosomal TLRs.PLoS One. 2013;8(2):e55264. doi: 10.1371/journal.pone.0055264. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405128 Free PMC article.
-
Toll-like receptor 2 (TLR2)-TLR9 crosstalk dictates IL-12 family cytokine production in microglia.Glia. 2012 Jan;60(1):29-42. doi: 10.1002/glia.21243. Epub 2011 Sep 7. Glia. 2012. PMID: 21901759 Free PMC article.
-
Oligodendroglia are limited in type I interferon induction and responsiveness in vivo.Glia. 2012 Oct;60(10):1555-66. doi: 10.1002/glia.22375. Epub 2012 Jun 26. Glia. 2012. PMID: 22736486 Free PMC article.
-
Protective effects of delayed intraventricular TLR7 agonist administration on cerebral white and gray matter following asphyxia in the preterm fetal sheep.Sci Rep. 2019 Jul 2;9(1):9562. doi: 10.1038/s41598-019-45872-y. Sci Rep. 2019. PMID: 31267031 Free PMC article.
-
Toll-like receptors 2, -3 and -4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1β release.Sci Rep. 2014 Oct 29;4:6824. doi: 10.1038/srep06824. Sci Rep. 2014. PMID: 25351234 Free PMC article.
References
-
- Berghofer B, Haley G, Frommer T, Bein G, Hackstein H. Natural and synthetic TLR7 ligands inhibit CpG-A- and CpG-C-oligodeoxynucleotide-induced IFN-alpha production. J Immunol. 2007;178:4072–4079. - PubMed
-
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. - PubMed
-
- Butchi NB, Pourciau S, Du M, Morgan TW, Peterson KE. Analysis of the neuroinflammatory response to TLR7 stimulation in the brain: comparison of multiple TLR7 and/or TLR8 agonists. J Immunol. 2008;180:7604–7612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

