Role of the receptor for advanced glycation end products in hepatic fibrosis
- PMID: 19998499
- PMCID: PMC2791271
- DOI: 10.3748/wjg.15.5789
Role of the receptor for advanced glycation end products in hepatic fibrosis
Abstract
Aim: To study the role of advanced glycation end products (AGE) and their specific receptor (RAGE) in the pathogenesis of liver fibrogenesis.
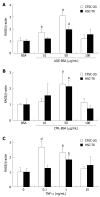
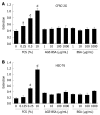
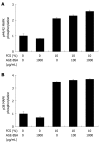
Methods: In vitro RAGE expression and extracellular matrix-related gene expression in both rat and human hepatic stellate cells (HSC) were measured after stimulation with the two RAGE ligands, advanced glycation end product-bovine serum albumin (AGE-BSA) and N(epsilon)-(carboxymethyl) lysine (CML)-BSA, or with tumor necrosis factor-alpha (TNF-alpha). In vivo RAGE expression was examined in models of hepatic fibrosis induced by bile duct ligation or thioacetamide. The effects of AGE-BSA and CML-BSA on HSC proliferation, signal transduction and profibrogenic gene expression were studied in vitro.
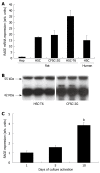
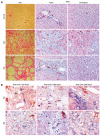
Results: In hepatic fibrosis, RAGE expression was enhanced in activated HSC, and also in endothelial cells, inflammatory cells and activated bile duct epithelia. HSC expressed RAGE which was upregulated after stimulation with AGE-BSA, CML-BSA, and TNF-alpha. RAGE stimulation with AGE-BSA and CML-BSA did not alter HSC proliferation, apoptosis, fibrogenic signal transduction and fibrosis- or fibrolysis-related gene expression, except for marginal upregulation of procollagen alpha1(I) mRNA by AGE-BSA.
Conclusion: Despite upregulation of RAGE in activated HSC, RAGE stimulation by AGE does not alter their fibrogenic activation. Therefore, RAGE does not contribute directly to hepatic fibrogenesis.
Figures
Similar articles
-
Increased Expression of S100B and RAGE in a Mouse Model of Bile Duct Ligation-induced Liver Fibrosis.J Korean Med Sci. 2021 Apr 12;36(14):e90. doi: 10.3346/jkms.2021.36.e90. J Korean Med Sci. 2021. PMID: 33847081 Free PMC article.
-
The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha.J Invest Dermatol. 2006 Feb;126(2):291-9. doi: 10.1038/sj.jid.5700070. J Invest Dermatol. 2006. PMID: 16374460
-
Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling.Lab Invest. 2014 May;94(5):503-16. doi: 10.1038/labinvest.2014.42. Epub 2014 Mar 10. Lab Invest. 2014. PMID: 24614199 Free PMC article.
-
Development and Progression of Non-Alcoholic Fatty Liver Disease: The Role of Advanced Glycation End Products.Int J Mol Sci. 2019 Oct 11;20(20):5037. doi: 10.3390/ijms20205037. Int J Mol Sci. 2019. PMID: 31614491 Free PMC article. Review.
-
RAGE and the pathogenesis of chronic kidney disease.Nat Rev Nephrol. 2010 Jun;6(6):352-60. doi: 10.1038/nrneph.2010.54. Epub 2010 Apr 27. Nat Rev Nephrol. 2010. PMID: 20421886 Review.
Cited by
-
Increased Expression of S100B and RAGE in a Mouse Model of Bile Duct Ligation-induced Liver Fibrosis.J Korean Med Sci. 2021 Apr 12;36(14):e90. doi: 10.3346/jkms.2021.36.e90. J Korean Med Sci. 2021. PMID: 33847081 Free PMC article.
-
Advanced Glycation End Products as a Predictor of Diabetes Mellitus in Chronic Hepatitis C-Related Cirrhosis.Front Med (Lausanne). 2020 Oct 26;7:588519. doi: 10.3389/fmed.2020.588519. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33195350 Free PMC article.
-
Increased liver AGEs induce hepatic injury mediated through an OST48 pathway.Sci Rep. 2017 Sep 25;7(1):12292. doi: 10.1038/s41598-017-12548-4. Sci Rep. 2017. PMID: 28947796 Free PMC article.
-
Ameliorative Effects of Nilotinib on CCl4 Induced Liver Fibrosis Via Attenuation of RAGE/HMGB1 Gene Expression and Oxidative Stress in Rat.Chonnam Med J. 2017 May;53(2):118-126. doi: 10.4068/cmj.2017.53.2.118. Epub 2017 May 25. Chonnam Med J. 2017. PMID: 28584790 Free PMC article.
-
Classical and alternative activation of rat hepatic sinusoidal endothelial cells by inflammatory stimuli.Exp Mol Pathol. 2013 Feb;94(1):160-7. doi: 10.1016/j.yexmp.2012.10.015. Epub 2012 Oct 24. Exp Mol Pathol. 2013. PMID: 23103612 Free PMC article.
References
-
- Schmidt AM, Yan SD, Stern DM. The dark side of glucose. Nat Med. 1995;1:1002–1004. - PubMed
-
- Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969;36:838–843. - PubMed
-
- Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. - PubMed
-
- Horiuchi S, Sakamoto Y, Sakai M. Scavenger receptors for oxidized and glycated proteins. Amino Acids. 2003;25:283–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

