Chemical and biological studies of nakiterpiosin and nakiterpiosinone
- PMID: 20000429
- PMCID: PMC2805047
- DOI: 10.1021/ja908626k
Chemical and biological studies of nakiterpiosin and nakiterpiosinone
Abstract
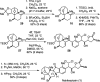
Nakiterpiosin and nakiterpiosinone are two related C-nor-D-homosteroids isolated from the sponge Terpios hoshinota that show promise as anticancer agents. We have previously described the asymmetric synthesis and revision of the relative configuration of nakiterpiosin. We now provide detailed information on the stereochemical analysis that supports our structure revision and the synthesis of the originally proposed and revised nakiterpiosin. In addition, we herein describe a refined approach for the synthesis of nakiterpiosin, the first synthesis of nakiterpiosinone, and preliminary mechanistic studies of nakiterpiosin's action in mammalian cells. Cells treated with nakiterpiosin exhibit compromised formation of the primary cilium, an organelle that functions as an assembly point for components of the Hedgehog signal transduction pathway. We provide evidence that the biological effects exhibited by nakiterpiosin are mechanistically distinct from those of well-established antimitotic agents such as taxol. Nakiterpiosin may be useful as an anticancer agent in those tumors resistant to existing antimitotic agents and those dependent on Hedgehog pathway responses for growth.
Figures



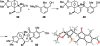






Similar articles
-
Nakiterpiosin targets tubulin and triggers mitotic catastrophe in human cancer cells.Mol Cancer Ther. 2010 Dec;9(12):3375-85. doi: 10.1158/1535-7163.MCT-10-0305. Epub 2010 Dec 7. Mol Cancer Ther. 2010. PMID: 21139045 Free PMC article.
-
Synthesis and structure revision of nakiterpiosin.J Am Chem Soc. 2009 Feb 4;131(4):1410-2. doi: 10.1021/ja808110d. J Am Chem Soc. 2009. PMID: 18998640 Free PMC article.
-
The Chemistry and Biology of Nakiterpiosin - C-nor-D-Homosteroids.Synlett. 2012 Oct;16(23):2298-2310. doi: 10.1055/s-0031-1290460. Synlett. 2012. PMID: 23226922 Free PMC article.
-
Recent advances of cytotoxic chalconoids targeting tubulin polymerization: Synthesis and biological activity.Eur J Med Chem. 2016 Oct 4;121:610-639. doi: 10.1016/j.ejmech.2016.05.067. Epub 2016 Jun 1. Eur J Med Chem. 2016. PMID: 27318983 Review.
-
Analysis of tubulin oligomers by analytical ultracentrifugation.Methods Cell Biol. 2010;95:275-88. doi: 10.1016/S0091-679X(10)95015-2. Methods Cell Biol. 2010. PMID: 20466140 Review.
Cited by
-
Naturally Occurring Organohalogen Compounds-A Comprehensive Review.Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review.
-
Steroids Bearing Heteroatom as Potential Drugs for Medicine.Biomedicines. 2023 Oct 3;11(10):2698. doi: 10.3390/biomedicines11102698. Biomedicines. 2023. PMID: 37893072 Free PMC article. Review.
-
Nakiterpiosin targets tubulin and triggers mitotic catastrophe in human cancer cells.Mol Cancer Ther. 2010 Dec;9(12):3375-85. doi: 10.1158/1535-7163.MCT-10-0305. Epub 2010 Dec 7. Mol Cancer Ther. 2010. PMID: 21139045 Free PMC article.
-
Natural products as inspiration for the development of new synthetic methods.J Chin Chem Soc. 2018 Jan;65(1):43-59. doi: 10.1002/jccs.201700134. Epub 2017 Aug 9. J Chin Chem Soc. 2018. PMID: 29430058 Free PMC article.
-
Visible light-promoted metal-free C-H activation: diarylketone-catalyzed selective benzylic mono- and difluorination.J Am Chem Soc. 2013 Nov 20;135(46):17494-500. doi: 10.1021/ja410815u. Epub 2013 Nov 12. J Am Chem Soc. 2013. PMID: 24180320 Free PMC article.
References
-
- Plucer-Rosario G. Coral Reefs. 1987;5:197–200.
- Rützler K, Smith KP. Sci. Mar. 1993;57:381–393.
- Rützler K, Smith KP. Sci. Mar. 1993;57:395–403.
- Liao M-H, Tang S-L, Hsu C-M, Wen K-C, Wu H, Chen W-M, Wang J-T, Meng P-J, Twan W-H, Lu C-K, Dai C-F, Soong K, Chen C-A. Zool. Stud. 2007;46:520.
- Lin W.-j., M.S. Thesis. National Sun Yat-sen University, Kaohsiung; Taiwan, R.O.C.: 2009.
-
- Teruya T, Nakagawa S, Koyama T, Suenaga K, Kita M, Uemura D. Tetreahedron Lett. 2003;44:5171–5173.
- Teruya T, Nakagawa S, Koyama T, Arimoto H, Kita M, Uemura D. Tetreahedron. 2004;60:6989–6993.
-
- Cooper MK, Porter JA, Young KE, Beachy PA. Science. 1998;280:1603–1607. - PubMed
- Incardona JP, Gaffield W, Kapur RP, Roelink H. Development. 1998;125:3553–3562. - PubMed
- Chen JK, Taipale J, Cooper MK, Beachy PA. Gen. Dev. 2002;16:2743–2748. - PMC - PubMed
- Wang Y, Zhou Z, Walsh CT, McMahon AP. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2623–2628. - PMC - PubMed
- Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3196–3201. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

