Mechanistic studies of human spermine oxidase: kinetic mechanism and pH effects
- PMID: 20000632
- PMCID: PMC2810717
- DOI: 10.1021/bi9017945
Mechanistic studies of human spermine oxidase: kinetic mechanism and pH effects
Abstract
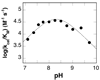
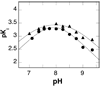
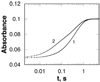
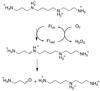
In mammalian cells, the flavoprotein spermine oxidase (SMO) catalyzes the oxidation of spermine to spermidine and 3-aminopropanal. Mechanistic studies have been conducted with the recombinant human enzyme. The initial velocity pattern in which the ratio between the concentrations of spermine and oxygen is kept constant establishes the steady-state kinetic pattern as ping-pong. Reduction of SMO by spermine in the absence of oxygen is biphasic. The rate constant for the rapid phase varies with the substrate concentration, with a limiting value (k(3)) of 49 s(-1) and an apparent K(d) value of 48 microM at pH 8.3. The rate constant for the slow step is independent of the spermine concentration, with a value of 5.5 s(-1), comparable to the k(cat) value of 6.6 s(-1). The kinetics of the oxidative half-reaction depend on the aging time after the spermine and enzyme are mixed in a double-mixing experiment. At an aging time of 6 s, the reaction is monophasic with a second-order rate constant of 4.2 mM(-1) s(-1). At an aging time of 0.3 s, the reaction is biphasic with two second-order constants equal to 4.0 and 40 mM(-1) s(-1). Neither is equal to the k(cat)/K(O(2)) value of 13 mM(-1) s(-1). These results establish the existence of more than one pathway for the reaction of the reduced flavin intermediate with oxygen. The k(cat)/K(M) value for spermine exhibits a bell-shaped pH profile, with an average pK(a) value of 8.3. This profile is consistent with the active form of spermine having three charged nitrogens. The pH profile for k(3) shows a pK(a) value of 7.4 for a group that must be unprotonated. The pK(i)-pH profiles for the competitive inhibitors N,N'-dibenzylbutane-1,4-diamine and spermidine show that the fully protonated forms of the inhibitors and the unprotonated form of an amino acid residue with a pK(a) of approximately 7.4 in the active site are preferred for binding.
Figures







Similar articles
-
Mechanistic studies of the yeast polyamine oxidase Fms1: kinetic mechanism, substrate specificity, and pH dependence.Biochemistry. 2010 Dec 14;49(49):10440-8. doi: 10.1021/bi1016099. Epub 2010 Nov 16. Biochemistry. 2010. PMID: 21067138 Free PMC article.
-
Mechanistic studies of mouse polyamine oxidase with N1,N12-bisethylspermine as a substrate.Biochemistry. 2005 May 10;44(18):7079-84. doi: 10.1021/bi050347k. Biochemistry. 2005. PMID: 15865452 Free PMC article.
-
pH dependence of a mammalian polyamine oxidase: insights into substrate specificity and the role of lysine 315.Biochemistry. 2009 Feb 24;48(7):1508-16. doi: 10.1021/bi802227m. Biochemistry. 2009. PMID: 19199575 Free PMC article.
-
Catabolism of polyamines.Amino Acids. 2004 Jun;26(3):217-33. doi: 10.1007/s00726-004-0070-z. Epub 2004 Apr 20. Amino Acids. 2004. PMID: 15221502 Review.
-
Spermine oxidase: ten years after.Amino Acids. 2012 Feb;42(2-3):441-50. doi: 10.1007/s00726-011-1014-z. Epub 2011 Aug 2. Amino Acids. 2012. PMID: 21809080 Review.
Cited by
-
Structure-function relationships in the evolutionary framework of spermine oxidase.J Mol Evol. 2013 Jun;76(6):365-70. doi: 10.1007/s00239-013-9570-3. Epub 2013 Jul 5. J Mol Evol. 2013. PMID: 23828398 Review.
-
Mechanistic and structural analyses of the roles of active site residues in yeast polyamine oxidase Fms1: characterization of the N195A and D94N enzymes.Biochemistry. 2012 Oct 30;51(43):8690-7. doi: 10.1021/bi3011434. Epub 2012 Oct 15. Biochemistry. 2012. PMID: 23034052 Free PMC article.
-
Mechanistic studies of the role of a conserved histidine in a mammalian polyamine oxidase.Arch Biochem Biophys. 2012 Dec 1;528(1):45-9. doi: 10.1016/j.abb.2012.08.007. Epub 2012 Aug 30. Arch Biochem Biophys. 2012. PMID: 22959971 Free PMC article.
-
Mechanistic and structural analyses of the role of His67 in the yeast polyamine oxidase Fms1.Biochemistry. 2012 Jun 19;51(24):4888-97. doi: 10.1021/bi300517s. Epub 2012 Jun 5. Biochemistry. 2012. PMID: 22642831 Free PMC article.
-
Mechanism of the Flavoprotein d-6-Hydroxynicotine Oxidase: Substrate Specificity, pH and Solvent Isotope Effects, and Roles of Key Active-Site Residues.Biochemistry. 2019 May 28;58(21):2534-2541. doi: 10.1021/acs.biochem.9b00297. Epub 2019 May 10. Biochemistry. 2019. PMID: 31046245 Free PMC article.
References
-
- Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4:781–792. - PubMed
-
- Seiler N. Catabolism of polyamines. Amino Acids. 2004;26:217–233. - PubMed
-
- Casero R, Jr, Pegg A. Spermidine/spermine N1-acetyltransferase--the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. - PubMed
-
- Seiler N, Peter M, Yu KFT, Alan AB. Polyamine oxidase, properties and functions. Prog. Brain Res. 1995;106:333–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

