New strategies for fluorescent probe design in medical diagnostic imaging
- PMID: 20000749
- PMCID: PMC3241938
- DOI: 10.1021/cr900263j
New strategies for fluorescent probe design in medical diagnostic imaging
Figures




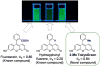
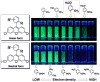
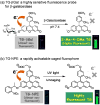


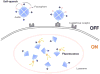



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

