Progressive spatial processing deficits in a mouse model of the fragile X premutation
- PMID: 20001115
- PMCID: PMC3410547
- DOI: 10.1037/a0017616
Progressive spatial processing deficits in a mouse model of the fragile X premutation
Abstract
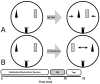
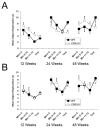
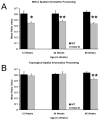
Fragile X associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder that is the result of a CGG trinucleotide repeat expansion in the range of 55-200 in the 5' UTR of the FMR1 gene. To better understand the progression of this disorder, a knock-in (CGG KI) mouse was developed by substituting the mouse CGG8 trinucleotide repeat with an expanded CGG98 repeat from human origin. It has been shown that this mouse shows deficits on the water maze at 52 weeks of age. In the present study, this CGG KI mouse model of FXTAS was tested on behavioral tasks that emphasize spatial information processing. The results demonstrate that at 12 and 24 weeks of age, CGG KI mice were unable to detect a change in the distance between two objects (metric task), but showed intact detection of a transposition of the objects (topological task). At 48 weeks of age, CGG KI mice were unable to detect either change in object location. These data indicate that hippocampal-dependent impairments in spatial processing may occur prior to parietal cortex-dependent impairments in FXTAS.
Figures

Similar articles
-
Spatiotemporal processing deficits in female CGG KI mice modeling the fragile X premutation.Behav Brain Res. 2012 Jul 15;233(1):29-34. doi: 10.1016/j.bbr.2012.04.029. Epub 2012 Apr 26. Behav Brain Res. 2012. PMID: 22561129 Free PMC article.
-
Temporal ordering deficits in female CGG KI mice heterozygous for the fragile X premutation.Behav Brain Res. 2010 Dec 1;213(2):263-8. doi: 10.1016/j.bbr.2010.05.010. Epub 2010 May 15. Behav Brain Res. 2010. PMID: 20478339 Free PMC article.
-
Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency.Hum Reprod. 2016 Jan;31(1):158-68. doi: 10.1093/humrep/dev280. Epub 2015 Nov 3. Hum Reprod. 2016. PMID: 26537920 Free PMC article.
-
Mouse models of fragile X-associated tremor ataxia.J Investig Med. 2009 Dec;57(8):837-41. doi: 10.2310/JIM.0b013e3181af59d6. J Investig Med. 2009. PMID: 19574928 Free PMC article. Review.
-
What has been learned from mouse models of the Fragile X Premutation and Fragile X-associated tremor/ataxia syndrome?Clin Neuropsychol. 2016 Aug;30(6):960-72. doi: 10.1080/13854046.2016.1158254. Epub 2016 Jun 29. Clin Neuropsychol. 2016. PMID: 27355912 Free PMC article. Review.
Cited by
-
A Majority of FXTAS Cases Present with Intranuclear Inclusions Within Purkinje Cells.Cerebellum. 2016 Oct;15(5):546-51. doi: 10.1007/s12311-016-0776-y. Cerebellum. 2016. PMID: 27108270
-
Neurodegeneration the RNA way.Prog Neurobiol. 2012 May;97(2):173-89. doi: 10.1016/j.pneurobio.2011.10.006. Epub 2011 Nov 3. Prog Neurobiol. 2012. PMID: 22079416 Free PMC article. Review.
-
Adaptation of the Arizona Cognitive Task Battery for use with the Ts65Dn mouse model (Mus musculus) of Down syndrome.J Comp Psychol. 2017 Aug;131(3):189-206. doi: 10.1037/com0000069. Epub 2017 Mar 23. J Comp Psychol. 2017. PMID: 28333487 Free PMC article.
-
A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis.Neurobiol Dis. 2011 Apr;42(1):85-98. doi: 10.1016/j.nbd.2011.01.008. Epub 2011 Jan 8. Neurobiol Dis. 2011. PMID: 21220020 Free PMC article.
-
A cross-sectional analysis of orienting of visuospatial attention in child and adult carriers of the fragile X premutation.J Neurodev Disord. 2014;6(1):45. doi: 10.1186/1866-1955-6-45. Epub 2014 Dec 11. J Neurodev Disord. 2014. PMID: 25937844 Free PMC article.
References
-
- Arocena DG, Iwahashi CK, Won N, Beilina A, Ludwig AL, Tassone F, et al. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Human Molecular Genetics. 2005;14(23):3661–3671. - PubMed
-
- Bontekoe CJ, de Graaff E, Nieuwenhuizen IM, Willemsen R, Oostra BA. FMR1 premutation allele (CGG)81 is stable in mice. European Journal of Human Genetics. 1997;5(5):293–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

