Early versus delayed fixed dose combination abacavir/lamivudine/zidovudine in patients with HIV and tuberculosis in Tanzania
- PMID: 20001518
- PMCID: PMC2858925
- DOI: 10.1089/aid.2009.0100
Early versus delayed fixed dose combination abacavir/lamivudine/zidovudine in patients with HIV and tuberculosis in Tanzania
Abstract
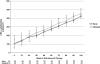
Fixed dose combination abacavir/lamivudine/zidovudine (ABC/3TC/ZDV) among HIV-1 and tuberculosis (TB)-coinfected patients was evaluated and outcomes between early vs. delayed initiation were compared. In a randomized, pilot study conducted in the Kilimanjaro Region of Tanzania, HIV-infected inpatients with smear-positive TB and total lymphocyte count <1200/mm(3) were randomized to initiate ABC/3TC/ZDV either 2 (early) or 8 (delayed) weeks after commencing antituberculosis therapy and were followed for 104 weeks. Of 94 patients screened, 70 enrolled (41% female, median CD4 count 103 cells/mm(3)), and 33 in each group completed 104 weeks. Two deaths and 12 serious adverse events (SAEs) were observed in the early arm vs. one death, one clinical failure, and seven SAEs in the delayed arm (p = 0.6012 for time to first grade 3/4 event, SAE, or death). CD4 cell increases were +331 and +328 cells/mm(3), respectively. TB-immune reconstitution inflammatory syndromes (TB-IRIS) were not observed in any subject. Using intent-to-treat (ITT), missing = failure analyses, 74% (26/35) vs. 89% (31/35) randomized to early vs. delayed therapy had HIV RNA levels <400 copies/ml at 104 weeks (p = 0.2182) and 66% (23/35) vs. 74% (26/35), respectively, had HIV RNA levels <50 copies/ml (p = 0.6026). In an analysis in which switches from ABC/3TC/ZDV = failure, those receiving early therapy were less likely to be suppressed to <400 copies/ml [60% (21/35) vs. 86% (30/35), p = 0.030]. TB-IRIS was not observed among the 70 coinfected subjects beginning antiretroviral treatment. ABC/3TC/ZDV was well tolerated and resulted in steady immunologic improvement. Rates of virologic suppression were similar between early and delayed treatment strategies with triple nucleoside regimens when substitutions were allowed.
Figures

Similar articles
-
Induction with abacavir/lamivudine/zidovudine plus efavirenz for 48 weeks followed by 48-week maintenance with abacavir/lamivudine/zidovudine alone in antiretroviral-naive HIV-1-infected patients.J Acquir Immune Defic Syndr. 2005 Jul 1;39(3):257-64. doi: 10.1097/01.qai.0000169664.15536.20. J Acquir Immune Defic Syndr. 2005. PMID: 15980684 Clinical Trial.
-
A randomized, double-blind study of triple nucleoside therapy of abacavir, lamivudine, and zidovudine versus lamivudine and zidovudine in previously treated human immunodeficiency virus type 1-infected children. The CNAA3006 Study Team.Pediatrics. 2001 Jan;107(1):E4. doi: 10.1542/peds.107.1.e4. Pediatrics. 2001. PMID: 11134468 Clinical Trial.
-
A randomized controlled trial of single-class maintenance therapy with abacavir/lamivudine/zidovudine after standard triple antiretroviral induction therapy: final 96-week results from the FREE study.HIV Med. 2015 Feb;16(2):122-31. doi: 10.1111/hiv.12186. Epub 2014 Dec 4. HIV Med. 2015. PMID: 25472825 Clinical Trial.
-
Zidovudine/Lamivudine vs. Abacavir/Lamivudine vs. Tenofovir/Emtricitabine in fixed-dose combinations as initial treatment for HIV patients: a systematic review and network meta-analysis.Colomb Med (Cali). 2017 Jun 30;48(2):70-81. Colomb Med (Cali). 2017. PMID: 29021641 Free PMC article.
-
Triple-nucleoside analog antiretroviral therapy: is there still a role in clinical practice? A review.MedGenMed. 2005 Jun 2;7(2):70. MedGenMed. 2005. PMID: 16369448 Free PMC article. Review.
Cited by
-
Bacteremic disseminated tuberculosis in sub-saharan Africa: a prospective cohort study.Clin Infect Dis. 2012 Jul;55(2):242-50. doi: 10.1093/cid/cis409. Epub 2012 Apr 16. Clin Infect Dis. 2012. PMID: 22511551 Free PMC article.
-
Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis.Clin Infect Dis. 2016 Oct 1;63(7):e147-e195. doi: 10.1093/cid/ciw376. Epub 2016 Aug 10. Clin Infect Dis. 2016. PMID: 27516382 Free PMC article.
-
Effects of time of initiation of antiretroviral therapy in the treatment of patients with HIV/TB co-infection: A systemic review and meta-analysis.Ann Med Surg (Lond). 2020 May 16;55:148-158. doi: 10.1016/j.amsu.2020.05.004. eCollection 2020 Jul. Ann Med Surg (Lond). 2020. PMID: 32477514 Free PMC article. Review.
-
What is the optimum time to start antiretroviral therapy in people with HIV and tuberculosis coinfection? A systematic review and meta-analysis.J Int AIDS Soc. 2021 Jul;24(7):e25772. doi: 10.1002/jia2.25772. J Int AIDS Soc. 2021. PMID: 34289243 Free PMC article.
-
Integration of antiretroviral therapy with tuberculosis treatment.N Engl J Med. 2011 Oct 20;365(16):1492-501. doi: 10.1056/NEJMoa1014181. N Engl J Med. 2011. PMID: 22010915 Free PMC article. Clinical Trial.
References
-
- World Health Organization. Global Tuberculosis Control: Surveillance, Planning, Financing. 2008. http://www.who.int/tb/publications/global_report/en/ [Aug 18;2009 ]. http://www.who.int/tb/publications/global_report/en/
-
- Ansari NA. Kombe AH. Kenyon TA, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis. 2002;6:55–63. - PubMed
-
- Corbett EL. Marston B. Churchyard GJ. De Cock KM. Tuberculosis in sub-Saharan Africa: Opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–937. - PubMed
-
- Grant AD. Djomand G. De Cock KM. Natural history and spectrum of disease in adults with HIV/AIDS in Africa. AIDS. 1997;11(Suppl B):S43–S54. - PubMed
-
- Ole-Nguyaine S. Crump JA. Kibiki GS, et al. HIV-associated morbidity, mortality, and diagnostic testing opportunities among inpatients at a referral hospital in northern Tanzania. Ann Trop Med Parasitol. 2004;98:171–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
