Targeting the transforming growth factor-beta signaling pathway in human cancer
- PMID: 20001556
- PMCID: PMC2796203
- DOI: 10.1517/13543780903382609
Targeting the transforming growth factor-beta signaling pathway in human cancer
Abstract
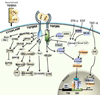
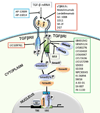
The transforming growth factor-ss (TGF-beta) signaling pathway plays a pivotal role in diverse cellular processes. TGF-beta switches its role from a tumor suppressor in normal or dysplastic cells to a tumor promoter in advanced cancers. It is widely believed that the Smad-dependent pathway is involved in TGF-beta tumor-suppressive functions, whereas activation of Smad-independent pathways, coupled with the loss of tumor-suppressor functions of TGF-beta, is important for its pro-oncogenic functions. TGF-beta signaling has been considered a useful therapeutic target. The discovery of oncogenic actions of TGF-beta has generated a great deal of enthusiasm for developing TGF-beta signaling inhibitors for the treatment of cancer. The challenge is to identify the group of patients where targeted tumors are not only refractory to TGF-beta-induced tumor suppressor functions but also responsive to the tumor-promoting effects of TGF-beta. TGF-beta pathway inhibitors, including small and large molecules, have now entered clinical trials. Preclinical studies with these inhibitors have shown promise in a variety of different tumor models. Here, we focus on the mechanisms of signaling and specific targets of the TGF-beta pathway that are critical effectors of tumor progression and invasion. This report also examines the therapeutic intervention of TGF-ss signaling in human cancers.
Figures
References
-
- Derynck R, Miyazono K. TGF-β and the TGF-β Family. In: Derynck R, Miyazono K, editors. The TGF-β Family. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 29–43.
-
- Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9(9):1000–1004. - PubMed
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF beta activation. Journal of Cell Science. 2003;116(2):217–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
