Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection
- PMID: 20001604
- PMCID: PMC2814432
- DOI: 10.1086/649227
Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection
Abstract
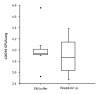
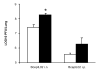
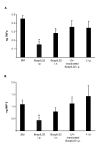
The therapeutic potential of bacteriophages (phages) in a mouse model of acute Burkholderia cenocepacia pulmonary infection was assessed. Phage treatment was administered by either intranasal inhalation or intraperitoneal injection. Bacterial density, macrophage inflammatory protein 2 (MIP-2), and tumor necrosis factor alpha (TNF-alpha) levels were significantly reduced in lungs of mice treated with intraperitoneal phages (P < .05). No significant differences in lung bacterial density or MIP-2 levels were found between untreated mice and mice treated with intranasal phages, intraperitoneal ultraviolet-inactivated phages, or intraperitoneal lambda phage control mice. Mock-infected mice treated with phage showed no significant increase in lung MIP-2 or TNF-alpha levels compared with mock-infected/mock-treated mice. We have demonstrated the efficacy of phage therapy in an acute B. cenocepacia lung infection model. Systemic phage administration was more effective than inhalational administration, suggesting that circulating phages have better access to bacteria in lungs than do topical phages.
Conflict of interest statement
Potential conflicts of interest: None of the authors has a commercial or other association that might pose a conflict of interest
Figures



References
-
- LiPuma JJ. Update on the Burkholderia cepacia complex. Curr Opin Pulm Med. 2005;11:528–33. - PubMed
-
- Courtney JM, Dunbar KE, McDowell A, et al. Clinical outcome of Burkholderia cepacia complex infection in cystic fibrosis adults. J Cyst Fibros. 2004;3:93–8. - PubMed
-
- Isles A, Maclusky I, Corey M, et al. Pseudomonas cepacia infection in cystic fibrosis: An emerging problem. J Pediatr. 1984;104:206–10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

