Three-dimensional culture alters primary cardiac cell phenotype
- PMID: 20001738
- PMCID: PMC2813151
- DOI: 10.1089/ten.tea.2009.0458
Three-dimensional culture alters primary cardiac cell phenotype
Abstract
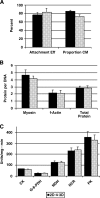
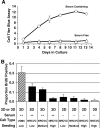
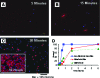
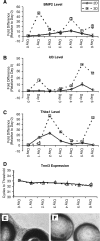
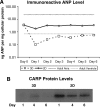
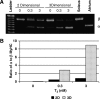
The directed formation of complex three-dimensional (3D) tissue architecture is a fundamental goal in tissue engineering and regenerative medicine. The growth of cells in 3D structures is expected to influence cellular phenotype and function, especially relative cell distribution, expression profiles, and responsiveness to exogenous signals; however, relatively few studies have been carried out to examine the effects of 3D reaggregation on cells from critical target organs, like the heart. Accordingly, we cultured primary cardiac ventricular cells in a 3D model system using a serum-free medium to test the hypothesis that expression profiles, multicellular organizational pathways, tissue maturation markers, and responsiveness to hormone stimulation were significantly altered in stable cell populations grown in 3D versus 2D culture. We found that distinct multi-cellular structures formed in 3D in conjunction with changes in mRNA expression profile, up-regulation of endothelial cell migratory pathways, decreases in the expression of fetal genes (Nppa and Ankrd1), and increased sensitivity to tri-iodothyronine stimulation when compared to parallel 2D cultures comprising the same cell populations. These results indicate that the culture of primary cardiac cells in 3D aggregates leads to physiologically relevant alterations in component cell phenotype consistent with cardiac ventricular tissue formation and maturation.
Figures


Similar articles
-
Impact of endothelial cells on 3D cultured smooth muscle cells in a biomimetic hydrogel.ACS Appl Mater Interfaces. 2012 Mar;4(3):1378-87. doi: 10.1021/am201648f. Epub 2012 Feb 22. ACS Appl Mater Interfaces. 2012. PMID: 22296557
-
3-D culture and endothelial cells improve maturity of human pluripotent stem cell-derived hepatocytes.Acta Biomater. 2019 Sep 1;95:371-381. doi: 10.1016/j.actbio.2019.07.047. Epub 2019 Jul 27. Acta Biomater. 2019. PMID: 31362140
-
Differentiation patterns of embryonic stem cells in two- versus three-dimensional culture.Cells Tissues Organs. 2013;197(5):399-410. doi: 10.1159/000346166. Epub 2013 Feb 9. Cells Tissues Organs. 2013. PMID: 23406658 Free PMC article.
-
Phenotype and gene expression of human mesenchymal stem cells in alginate scaffolds.Tissue Eng Part A. 2009 Jul;15(7):1763-73. doi: 10.1089/ten.tea.2008.0306. Tissue Eng Part A. 2009. PMID: 19115828
-
Modeling Physiological Events in 2D vs. 3D Cell Culture.Physiology (Bethesda). 2017 Jul;32(4):266-277. doi: 10.1152/physiol.00036.2016. Physiology (Bethesda). 2017. PMID: 28615311 Free PMC article. Review.
Cited by
-
The multiparametric effects of hydrodynamic environments on stem cell culture.Tissue Eng Part B Rev. 2011 Aug;17(4):249-62. doi: 10.1089/ten.TEB.2011.0040. Epub 2011 May 25. Tissue Eng Part B Rev. 2011. PMID: 21491967 Free PMC article. Review.
-
Multi-cellular interactions sustain long-term contractility of human pluripotent stem cell-derived cardiomyocytes.Am J Transl Res. 2014 Nov 22;6(6):724-35. eCollection 2014. Am J Transl Res. 2014. PMID: 25628783 Free PMC article.
-
Advances in cell culture: anchorage dependence.Philos Trans R Soc Lond B Biol Sci. 2015 Feb 5;370(1661):20140040. doi: 10.1098/rstb.2014.0040. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 25533097 Free PMC article. Review.
-
The expanding world of tissue engineering: the building blocks and new applications of tissue engineered constructs.IEEE Rev Biomed Eng. 2013;6:47-62. doi: 10.1109/RBME.2012.2233468. Epub 2012 Dec 20. IEEE Rev Biomed Eng. 2013. PMID: 23268388 Free PMC article. Review.
-
2D and 3D-organized cardiac cells shows differences in cellular morphology, adhesion junctions, presence of myofibrils and protein expression.PLoS One. 2012;7(5):e38147. doi: 10.1371/journal.pone.0038147. Epub 2012 May 25. PLoS One. 2012. PMID: 22662278 Free PMC article.
References
-
- Jakab K. Norotte C. Damon B. Marga F. Neagu A. Besch-Williford C.L. Kachurin A. Church K.H. Park H. Mironov V. Markwald R. Vunjak-Novakovic G. Forgacs G. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng Part A. 2008;14:413. - PubMed
-
- Yildirim Y. Naito H. Didie M. Karikkineth B.C. Biermann D. Eschenhagen T. Zimmermann W.H. Development of a biological ventricular assist device: preliminary data from a small animal model. Circulation. 2007;116:I16. - PubMed
-
- Masuda S. Shimizu T. Yamato M. Okano T. Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev. 2008;60:277. - PubMed
-
- Sekine H. Shimizu T. Hobo K. Sekiya S. Yang J. Yamato M. Kurosawa H. Kobayashi E. Okano T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145. - PubMed
-
- Akins R. Gratton K. Quezada E. Rutter H. Tsuda T. Soteropoulos P. Gene expression profile of bioreactor-cultured cardiac cells: activation of morphogenetic pathways for tissue engineering. DNA Cell Biol. 2007;26:425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

