Factors influencing time to vancomycin-induced clearance of nonendocarditis methicillin-resistant Staphylococcus aureus bacteremia: role of platelet microbicidal protein killing and agr genotypes
- PMID: 20001853
- PMCID: PMC2819315
- DOI: 10.1086/649429
Factors influencing time to vancomycin-induced clearance of nonendocarditis methicillin-resistant Staphylococcus aureus bacteremia: role of platelet microbicidal protein killing and agr genotypes
Abstract
Background: Vancomycin susceptibility, the accessory gene global regulator (agr) genotype and function, staphylococcal cassette chromosome (SCC) mec type, and susceptibility to cationic thrombin-induced platelet microbicidal protein 1 (tPMP-1) have been individually predictive of duration of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. This investigation evaluated the interrelationship of these factors with time to clearance of MRSA bacteremia during vancomycin therapy in patients without endocarditis.
Methods: Vancomycin minimum inhibitory concentration and in vitro killing, agr function (delta-hemolysin activity), agr group, SCCmec type, and survival in tPMP-1 killing assays were determined for 29 MRSA bacteremia isolates.
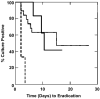
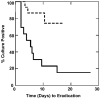
Results: Increased resistance to tPMP-1 killing was observed with agr group III MRSA (P = .025) and MRSA with reduced or absent agr function (P = .023). The median time to clearance of MRSA bacteremia was earlier for agr group III (3 days) versus group I (10.5 days) or II (15 days) (P = .001). In multivariate analysis, agr group II, reduced tPMP-1 killing in vitro, and prior vancomycin exposure were significant independent predictors of longer MRSA bacteremia duration.
Conclusions: Specific genotypic, phenotypic, and clinical parameters appear to correlate with persistent MRSA bacteremia. The interrelationship of these and other factors probably contributes to vancomycin-mediated clearance of MRSA bacteremia.
Conflict of interest statement
Potential conflicts of interest: P.A.M. is currently employed by Cubist Pharmaceuticals. A.F. has received research funding from Theravance and Wyeth Pharmaceuticals. AS.B. has received research funding from Cubist Pharmaceuticals, Ortho-McNeil Pharmaceuticals, Novozyme Pharmaceuticals, Cerexa Pharmaceuticals, and Targanta Pharmaceuticals and is currently on the Cubist Scientific Advisory Board. M.R.Y. has received research funding from Pfizer, Novozyme Pharmaceuticals, Cubist Pharmaceuticals, and Amgen and is a cofounder and shareholder of NovaDigm Therapeutics. G.S. has received research funding from Cubist and Pfizer Pharmaceuticals; has been a consultant for Cubist, Pfizer, and Ortho McNeil; and has been on the speakers’ bureaus for Cubist, Pfizer, and Wyeth Pharmaceuticals.
Figures


References
-
- Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering RC., Jr Agr group II genetic polymorphism in methicillin-resistant Staphylococcus aureus predicting failure of vancomycin therapy. Clin Infect Dis. 2004;38:1700–5. - PubMed
-
- Sakoulas G, Moise-Broder PA, Schentag JJ, Forrest A, Moellering RC, Jr, Eliopoulos GM. Relationship of vancomycin minimum inhibitory concentration (MIC) and bactericidal activity to the treatment efficacy of vancomycin in methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–402. - PMC - PubMed
-
- Hidayat LK, Hsu DI, Quist R, et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166:2138–44. - PubMed
-
- Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

