Caught in the middle: the role of Bag3 in disease
- PMID: 20001957
- PMCID: PMC7291699
- DOI: 10.1042/BJ20091739
Caught in the middle: the role of Bag3 in disease
Abstract
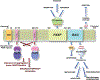
Bag3 is a Bag family co-chaperone that regulates the ATPase activity of Hsp70 (heat-shock protein 70) chaperones. Recent studies have demonstrated that Bag3 can initiate macroautophagy in co-operation with small heat-shock protein HspB8. In this issue of the Biochemical Journal, Fuchs and co-workers have discovered the IPV motif in Bag3 that is necessary for binding to HspB8. The authors have also identified HspB6 as a new binding partner for Bag3 and characterized further the binding of both HspB8 and HspB6 in Bag3-mediated clearance of aggregated polyglutamine-containing protein Htt43Q (huntingtin exon 1 fragment with 43 CAG repeats). It is clear from recent identification of a Bag3 mutation that causes a form of muscular dystrophy that the full function of Bag3 in disease is not clear. We will apply the findings of Fuchs et al. in this issue to reconcile the phenotypes of Bag3 homologue knockouts with the emerging role of Bag3 in autophagy.
Figures
Comment on
-
Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction.Biochem J. 2009 Dec 14;425(1):245-55. doi: 10.1042/BJ20090907. Biochem J. 2009. PMID: 19845507
References
-
- Doong H, Vrailas A and Kohn EC (2002) What’s in the ‘BAG’? – A functional domain analysis of the BAG-family proteins. Cancer Lett. 188, 25–32 - PubMed
-
- Fuchs M, Poirier DJ, Seguin S, Lambert H, Carra S, Charrette SJ and Landry J (2010) Identification of the key structural motifs involved in HspB8/HspB6–Bag3 interaction. Biochem. J. 425, 245–255 - PubMed
-
- Doong H, Price J, Kim YS, Gasbarre C, Probst J, Liotta LA, Blanchette J, Rizzo K and Kohn E (2000) CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-γ and Hsp70/Hsc70. Oncogene 19, 4385–4395 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

