Rise in antiobesity drug prescribing for children and adolescents in the UK: a population-based study
- PMID: 20002078
- PMCID: PMC2810795
- DOI: 10.1111/j.1365-2125.2009.03528.x
Rise in antiobesity drug prescribing for children and adolescents in the UK: a population-based study
Abstract
What is already known about this subject: * The antiobesity drugs sibutramine and orlistat are not licensed for use in children and adolescents in the UK or USA. * Clinical trials suggest antiobesity drugs are effective and well-tolerated in obese adolescents.
What this study adds: * Prescribing of unlicensed antiobesity drugs in children and adolescents has increased significantly in the past 8 years. * Most prescribed antiobesity drugs in children and adolescents are rapidly discontinued before patients can see clinical benefit, suggesting they are poorly tolerated or poorly efficacious.
Aims: The international childhood obesity epidemic has driven increased use of unlicensed antiobesity drugs, whose efficacy and safety are poorly studied in children and adolescents. We investigated the use of unlicensed antiobesity drugs (orlistat, sibutramine and rimonabant) in children and adolescents (0-18 years) in the UK.
Methods: Population-based prescribing data from the UK General Practice Research Database between 1 January 1999 and 31 December 2006.
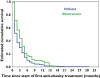
Results: A total of 452 subjects received 1334 prescriptions during the study period. The annual prevalence of antiobesity drug prescriptions rose significantly from 0.006 per 1000 [95% confidence interval (CI) 0.0007, 0.0113] in 1999 to 0.091 per 1000 (95% CI 0.07, 0.11) in 2006, a 15-fold increase, with similar increases seen in both genders. The majority of prescriptions were made to those >or=14 years old, although 25 prescriptions were made for children <12 years old. Orlistat accounted for 78.4% of all prescriptions; only one patient was prescribed rimonabant. However, approximately 45% of the patients ceased orlistat and 25% ceased sibutramine after only 1 month. The estimated mean treatment durations for orlistat and sibutramine were 3 and 4 months, respectively.
Conclusions: Prescribing of unlicensed antiobesity drugs in children and adolescents has dramatically increased in the past 8 years. The majority are rapidly discontinued before patients can see weight benefit, suggesting they are poorly tolerated or poorly efficacious when used in the general population. Further research into the effectiveness and safety of antiobesity drugs in clinical populations of children and adolescents is needed.
Figures
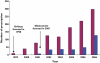
 ); Sibutramine (
); Sibutramine ( )
)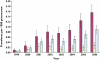
 ); boys (
); boys ( ); overall (
); overall ( )
)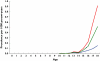
 ); boys (
); boys ( ); overall (
); overall ( )
)
References
-
- Lobstein T, Leach RJ. Tackling Obesities: Future Choices—International Comparisons of Obesity Trends, Determinants and Responses—Evidence Review -2 Children. 2nd edn. London: Government Office for Science; 2007.
-
- Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med. 1994;154:1842–7. - PubMed
-
- Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108:712–8. - PubMed
-
- Cross-Government Obesity Unit DoHDoCSaF. Healthy Weight, Healthy Lives: a Cross-Government Strategy for England. London: HM Govermment; 2008.
-
- McPherson K, Marsh T, Brown M. Tackling Obesities: Future Choices – Modelling Future Trends in Obesity & Their Impact on Health. 2nd edn. London: Government Office for Science; 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

