Pharmacokinetics and pharmacodynamics of LC15-0444, a novel dipeptidyl peptidase IV inhibitor, after multiple dosing in healthy volunteers
- PMID: 20002082
- PMCID: PMC2810799
- DOI: 10.1111/j.1365-2125.2009.03376.x
Pharmacokinetics and pharmacodynamics of LC15-0444, a novel dipeptidyl peptidase IV inhibitor, after multiple dosing in healthy volunteers
Abstract
What is already known about this subject: * The importance of efficient drug development using biomarkers has been increasingly emphasized, from preclinical studies to clinical trials. * However, as yet few validated or qualified biomarkers are used in early-stage drug development in terms of clinical pharmacology and disease pathophysiology.
What this study adds: * This first-time-in-human study provides evidence of the pharmacological activity of LC15-0444 in humans, by using dipeptidyl peptidase IV activity and active glucagon-like peptide-1 concentrations. * LC15-0444 possesses pharmacokinetic and pharmacodynamic characteristics that support a once-daily dosing regimen.
Aims: LC15-0444 is a selective and competitive inhibitor of dipeptidyl peptidase (DPP) IV with potential for the treatment of Type 2 diabetes. The aim was to investigate the pharmacokinetic (PK) and pharmacodynamic (PD) profiles after multiple oral ascending doses of LC15-0444 in healthy male subjects.
Methods: A dose block-randomized, double-blind, placebo-controlled, parallel group study was performed in three groups with 10 subjects (eight for active drug; two for placebo) per group; each group received 200, 400 or 600 mg of LC15-0444 once daily for 10 days. Blood and urine samples were collected up to 24 h after the first dosing and up to 72 h after the last dosing.
Results: The LC15-0444 concentration-time profiles exhibited characteristics of multicompartment disposition. No dose- or time-dependent change in PK parameters was observed. Mean elimination half-life was in a range 16.6-20.1 h in the dose groups. Mean renal clearance and fraction of unchanged drug excreted in urine was 18.6-21.9 and 0.40-0.48 l h(-1), respectively. In the steady state, mean accumulation ratios by dose groups were between 1.22 and 1.31. More than 80% inhibition of DPP IV activity from baseline was sustained for >24 h in all dose groups.
Conclusions: This study provides evidence of the pharmacological activity of LC15-0444 in humans. LC15-0444 possesses PK and PD characteristics that support a once-daily dosing regimen.
Figures
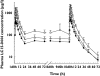
 )
)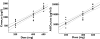
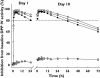
 ); 400 mg (N = 8) (—▵—); 600 mg (N = 8) (
); 400 mg (N = 8) (—▵—); 600 mg (N = 8) ( )
)
 ); 200 mg (N = 8) (
); 200 mg (N = 8) ( ); 400 mg (N = 8) (
); 400 mg (N = 8) ( ); 600 mg (N = 8) (
); 600 mg (N = 8) ( )
)References
-
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. - PubMed
-
- Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–40. - PubMed
-
- Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, Forgue ST. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17:1278–83. - PubMed
-
- Krikken JA, Lely AT, Bakker SJ, Navis G. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int. 2007;71:260–5. - PubMed
-
- Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K, Marsilio F, McCann ME, Patel RA, Petrov A, Scapin G, Patel SB, Roy RS, Wu JK, Wyvratt MJ, Zhang BB, Zhu L, Thornberry NA, Weber AE. 2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48:141–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

