Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype
- PMID: 20002085
- PMCID: PMC2810802
- DOI: 10.1111/j.1365-2125.2009.03534.x
Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype
Abstract
What is already known about this subject: * Pharmacokinetic variability of voriconazole is largely caused by CYP3A4- and CYP2C19-mediated metabolism. * Oral bioavailability of voriconazole has been claimed to be almost 100%, thus facilitating a change from intravenous to oral application without dose adjustment.
What this study adds: * For the first time voriconazole exposure after intravenous and oral administration in relation to CYP2C19 activity is reported. * In addition, the predominant metabolic pathway is the hydroxylation that seems to be influenced by the CYP2C19 genotype. * Enterohepatic circulation of both hydroxylated metabolites must be anticipated.
Aims: The aim was to determine the pharmacokinetics of voriconazole after a single oral dose in comparison with intravenous (i.v.) administration in healthy individuals stratified according to the cytochrome P450 (CYP) 2C19 genotype. In addition, the possible metabolic pathways and their modulation according to CYP2C19 genotype were investigated after oral and i.v. administration of voriconazole.
Methods: In a single-centre, open-label, two-period crossover study 20 participants received single doses of 400 mg voriconazole orally and 400 mg voriconazole intravenously in randomized order. Blood and urine samples were collected up to 96 h post dose and the voriconazole and three major metabolites were quantified by high-performance liquid chromatography coupled to mass spectroscopy.
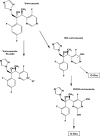
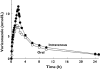
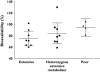
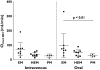
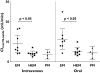
Results: Absolute oral bioavailability of voriconazole was 82.6% (74.1, 91.0). It ranged from 94.4% (78.8, 109.9) in CYP2C19 poor metabolizers to 75.2% (62.9, 87.4) in extensive metabolizers. In contrast to voriconazole and its N-oxide, the plasma concentrations of both hydroxylated metabolites showed a large second peak after 24 h. Independent of the route of administration, voriconazole partial metabolic hydroxylation after i.v. administration was eightfold higher compared with N-oxidation [48.8 ml min(-1) (30.5, 67.1) vs. 6.1 ml min(-1) (4.1, 8.0)]. The formation of the metabolites was related to CYP2C19 activity.
Conclusions: Independent of the route of administration, voriconazole exposure was three times higher in CYP2C19 poor metabolizers compared with extensive metabolizers. Voriconazole has a high bioavailability with no large differences between the CYP2C19 genotypes. The hydroxylation pathway of voriconazole elimination exceeded the N-oxidation, both influenced by the CYP2C19 genotype.
Figures



References
-
- Fachinformation VFEND. FachInfo. Fachinformationsverzeichnis. Deutschland Ausgabe 2008/6: BPI Service Gmbh.
-
- Ghannoum MA, Kuhn DM. Voriconazole – better chances for patients with invasive mycoses. Eur J Med Res. 2002;7:242–56. - PubMed
-
- Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31:540–7. - PubMed
-
- Weiss J, ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, Haefeli WE, Mikus G. CYP2C19 genotype is a major factor contributing to the highly variably pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49:196–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

