Inhibition of oral midazolam clearance by boosting doses of ritonavir, and by 4,4-dimethyl-benziso-(2H)-selenazine (ALT-2074), an experimental catalytic mimic of glutathione oxidase
- PMID: 20002087
- PMCID: PMC2810804
- DOI: 10.1111/j.1365-2125.2009.03545.x
Inhibition of oral midazolam clearance by boosting doses of ritonavir, and by 4,4-dimethyl-benziso-(2H)-selenazine (ALT-2074), an experimental catalytic mimic of glutathione oxidase
Abstract
What is already known about this subject: * The viral protease inhibitor ritonavir is known to inhibit clearance of intravenous midazolam. * ALT-2074, a catalytic mimic of glutathione oxidase, inhibits human cytochrome P450 3A (CYP3A) isoforms in vitro.
What this study adds: * Short-term administration of low-dose ritonavir increases area under the plasma concentration curve following oral midazolam by a factor of 28. * Therefore ritonavir is an appropriate positive control inhibitor for clinical drug interaction studies involving CYP3A substrates. * Midazolam clearance is weakly inhibited by ALT-2074, consistent with its in vitro profile.
Aims: We evaluated whether 'boosting' doses of ritonavir can serve as a positive control inhibitor for pharmacokinetic drug-drug interaction studies involving cytochrome P450 3A (CYP3A). The study also determined whether 4,4-dimethyl-benziso-(2H)-selenazine (ALT-2074), an investigational organoselenium compound that acts as a catalytic mimic of glutathione oxidase, inhibits CYP3A metabolism in vivo.
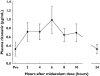
Methods: Thirteen healthy volunteers received single 3-mg oral doses of midazolam on three occasions: in the control condition, during co-treatment with low-dose ritonavir (three oral doses of 100 mg over 24 h), and during co-treatment with ALT-2074 (three oral doses of 80 mg over 24 h).
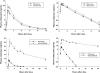
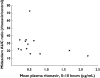
Results: Ritonavir increased mean (+/-SE) total area under the curve (AUC) for midazolam by a factor of 28.4 +/- 4.2 (P < 0.001), and reduced oral clearance to 4.2 +/- 0.5% of control (P < 0.001). In contrast, ALT-2074 increased midazolam AUC by 1.25 +/- 0.11 (P < 0.05), and reduced oral clearance to 88 +/- 8% of control.
Conclusions: Low-dose ritonavir produces extensive CYP3A inhibition exceeding that of ketoconazole (typically 10- to 15-fold midazolam AUC enhancement), and is a suitable positive control index inhibitor for drug-drug interaction studies. ALT-2074 inhibits CYP3A metabolism to a small degree that is of uncertain clinical importance.
Figures
References
-
- Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol. 1989;36:89–96. - PubMed
-
- Perloff MD, von Moltke LL, Court MH, Kotegawa T, Shader RI, Greenblatt DJ. Midazolam and triazolam biotransformation in mouse and human liver microsomes: relative contribution of CYP3A and CYP2C isoforms. J Pharmacol Exp Ther. 2000;292:618–28. - PubMed
-
- von Moltke LL, Greenblatt DJ, Schmider J, Duan SX, Wright CE, Harmatz JS, Shader RI. Midazolam hydroxylation by human liver microsomes in vitro: inhibition by fluoxetine, norfluoxetine, and by azole antifungal agents. J Clin Pharmacol. 1996;36:783–91. - PubMed
-
- Huang SM, Temple R, Throckmorton DC, Lesko LJ. Drug interaction studies: study design, data analysis, and implications for dosing and labeling. Clin Pharmacol Ther. 2007;81:298–304. - PubMed
-
- Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA. The conduct of in vitro and in vivo drug–drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31:815–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

