An alpha-amylase is a novel receptor for Bacillus thuringiensis ssp. israelensis Cry4Ba and Cry11Aa toxins in the malaria vector mosquito Anopheles albimanus (Diptera: Culicidae)
- PMID: 20002140
- PMCID: PMC3721636
- DOI: 10.1111/j.1462-2920.2009.02117.x
An alpha-amylase is a novel receptor for Bacillus thuringiensis ssp. israelensis Cry4Ba and Cry11Aa toxins in the malaria vector mosquito Anopheles albimanus (Diptera: Culicidae)
Abstract
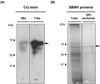
Bacillus thuringiensis ssp. israelensis (Bti) produces four Cry toxins (Cry4Aa, Cry4Ba, Cry10Aa and Cry11Aa), and two Cyt proteins (Cyt1Aa and Cyt2Ba), toxic to mosquito-larvae of the genus Aedes, Anopheles and Culex, important human disease vectors that transmit dengue virus, malaria and filarial parasites respectively. Previous work showed that Bti is highly toxic to Anopheles albimanus, the main vector for transmission of malaria in Mexico. In this work, we analysed the toxicity of isolated Cry proteins of Bti and identified an An. albimanus midgut protein as a putative Cry4Ba and Cry11Aa receptor molecule. Biossays showed that Cry4Ba and Cry11Aa of Bti are toxic to An. albimanus larvae. Ligand blot assays indicated that a 70 kDa glycosylphosphatidylinositol-anchored protein present in midgut brush border membrane vesicles of An. albimanus interacts with Cry4Ba and Cry11Aa toxins. This protein was identified as an alpha-amylase by mass spectrometry and enzymatic activity assays. The cDNA that codes for the alpha-amylase was cloned by means of 5'- and 3'-RACE experiments. Recombinant alpha-amylase expressed in Escherichia coli specifically binds Cry4Ba and Cry11Aa toxins.
Figures


 ) and an asterisk (*) respectively. Peptide sequences obtained from LC-MS/MS identification are underlined. The N-terminal hydrophobic domain that corresponds to the putative signal peptide is shown in lower case. The ω-site for GPI modification and the amino acid putatively implicated in the cleavage site is shown as a pentagon. Asparagines predicted to be N-glycosylated are encircled. Seven conserved regions (I to VII) characteristic of the α-amylase family proteins are boxed. The catalytically important residues, the histidines responsible for substrate binding and the amino acids involved in binding of calcium ions are referred by diamonds, squares or a double underline respectively.The GenBank accession number of aamy1 gene is GQ344953.
) and an asterisk (*) respectively. Peptide sequences obtained from LC-MS/MS identification are underlined. The N-terminal hydrophobic domain that corresponds to the putative signal peptide is shown in lower case. The ω-site for GPI modification and the amino acid putatively implicated in the cleavage site is shown as a pentagon. Asparagines predicted to be N-glycosylated are encircled. Seven conserved regions (I to VII) characteristic of the α-amylase family proteins are boxed. The catalytically important residues, the histidines responsible for substrate binding and the amino acids involved in binding of calcium ions are referred by diamonds, squares or a double underline respectively.The GenBank accession number of aamy1 gene is GQ344953.
 ) and an asterisk (*) respectively. Peptide sequences obtained from LC-MS/MS identification are underlined. The N-terminal hydrophobic domain that corresponds to the putative signal peptide is shown in lower case. The ω-site for GPI modification and the amino acid putatively implicated in the cleavage site is shown as a pentagon. Asparagines predicted to be N-glycosylated are encircled. Seven conserved regions (I to VII) characteristic of the α-amylase family proteins are boxed. The catalytically important residues, the histidines responsible for substrate binding and the amino acids involved in binding of calcium ions are referred by diamonds, squares or a double underline respectively.The GenBank accession number of aamy1 gene is GQ344953.
) and an asterisk (*) respectively. Peptide sequences obtained from LC-MS/MS identification are underlined. The N-terminal hydrophobic domain that corresponds to the putative signal peptide is shown in lower case. The ω-site for GPI modification and the amino acid putatively implicated in the cleavage site is shown as a pentagon. Asparagines predicted to be N-glycosylated are encircled. Seven conserved regions (I to VII) characteristic of the α-amylase family proteins are boxed. The catalytically important residues, the histidines responsible for substrate binding and the amino acids involved in binding of calcium ions are referred by diamonds, squares or a double underline respectively.The GenBank accession number of aamy1 gene is GQ344953.
References
-
- Bayyareddy K, Andacht TM, Abdullah MA, Adang MJ. Proteomic identification of Bacillus thuringiensis subsp. israelensis toxin Cry4Ba binding proteins in midgut membranes from Aedes (Stegomyia) aegypti Linnaeus (Diptera, Culicidae) larvae. Insect Biochem Mol Biol. 2009;39:279–286. - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

