Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6
- PMID: 20002448
- PMCID: PMC2819495
- DOI: 10.1111/j.1365-2249.2009.04069.x
Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6
Abstract
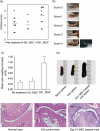
The immunomodulatory ability of mesenchymal stem cells (MSCs) may be used to develop therapies for autoimmune diseases. Flk-1(+) MSCs are a population of MSCs with defined phenotype and their safety has been evaluated in Phase 1 clinical trials. We designed this study to evaluate whether Flk-1(+) MSCs conferred a therapeutic effect on collagen-induced arthritis (CIA), an animal model of rheumatic arthritis, and to explore the underlying mechanisms. Flk-1(+) MSCs, 1-2 x 10(6), were injected into CIA mice on either day 0 or day 21. The clinical course of arthritis was monitored. Serum cytokine profile was determined by cytometric bead array kit or enzyme-linked immunosorbent assay. Flk-1(+) MSCs and splenocytes co-culture was conducted to explore the underlying mechanisms. Flk-1(+) MSCs did not confer therapeutic benefits. Clinical symptom scores and histological evaluation suggested aggravation of arthritis in mice treated with MSCs at day 21. Serum cytokine profile analysis showed marked interleukin (IL)-6 secretion immediately after MSC administration. Results of in vitro culture of splenocytes confirmed that the addition of Flk-1(+) MSCs promoted splenocyte proliferation and increased IL-6 and IL-17 secretion. Moreover, splenocyte proliferation was also enhanced in mice treated with MSCs at day 21. Accordingly, MSCs at low concentrations were found to promote lipopolysaccharide-primed splenocytes proliferation in an in vitro co-culture system. We propose that Flk-1(+) MSCs aggravate arthritis in CIA model by at least up-regulating secretion of IL-6, which favours Th17 differentiation. When Flk-1(+) MSCs are used for patients, we should be cautious about subjects with rheumatoid arthritis.
Figures

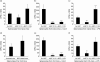



References
-
- Fang B, Liao L, Shi M, Yang S, Zhao RC. Multipotency of Flk1CD34 progenitors derived from human fetal bone marrow. J Lab Clin Med. 2004;143:230–40. - PubMed
-
- Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–9. - PubMed
-
- Deng W, Han Q, Liao L, et al. Engrafted bone marrow-derived flk-(1+) mesenchymal stem cells regenerate skin tissue. Tissue Eng. 2005;11:110–19. - PubMed
-
- Liu Y, Yan X, Sun Z, et al. Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev. 2007;16:695–706. - PubMed
-
- Bartholomew A, Patil S, Mackay A, et al. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum Gene Ther. 2001;12:1527–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

