Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot
- PMID: 20002787
- PMCID: PMC2842495
- DOI: 10.1111/j.1365-2567.2009.03200.x
Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot
Abstract
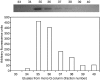
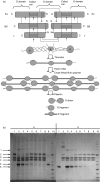
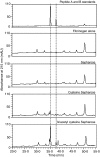
The lectin pathway of complement is activated upon binding of mannan-binding lectin (MBL) or ficolins (FCNs) to their targets. Upon recognition of targets, the MBL-and FCN-associated serine proteases (MASPs) are activated, allowing them to generate the C3 convertase C4b2a. Recent findings indicate that the MASPs also activate components of the coagulation system. We have previously shown that MASP-1 has thrombin-like activity whereby it cleaves and activates fibrinogen and factor XIII. MASP-2 has factor Xa-like activity and activates prothrombin through cleavage to form thrombin. We now report that purified L-FCN-MASPs complexes, bound from serum to N-acetylcysteine-Sepharose, or MBL-MASPs complexes, bound to mannan-agarose, generate clots when incubated with calcified plasma or purified fibrinogen and factor XIII. Plasmin digestion of the clot and analysis using anti-D-dimer antibodies revealed that the clot was made up of fibrin and was similar to that generated by thrombin in normal human plasma. Fibrinopeptides A and B (FPA and FPB, respectively) were released after fibrinogen cleavage by L-FCN-MASPs complexes captured on N-acetylcysteine-Sepharose. Studies of inhibition of fibrinopeptide release indicated that the dominant pathway for clotting catalysed by the MASPs is via MASP-2 and prothrombin activation, as hirudin, a thrombin inhibitor that does not inhibit MASP-1 and MASP-2, substantially inhibits fibrinopeptide release. In the light of their potent chemoattractant effects on neutrophil and fibroblast recruitment, the MASP-mediated release of FPA and FPB may play a role in early immune activation. Additionally, MASP-catalysed deposition and polymerization of fibrin on the surface of micro-organisms may be protective by limiting the dissemination of infection.
Figures







References
-
- Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–53. - PubMed
-
- Gal P, Dobo J, Zavodszky P, Sim RB. Early complement proteases: C1r, C1s and MASPs. A structural insight into activation and functions. Mol Immunol. 46:2745–52. - PubMed
-
- Mayilyan KR, Presanis JS, Arnold JN, Hajela K, Sim RB. Heterogeneity of MBL- MASP complexes. Mol Immunol. 2006;43:1286–92. - PubMed
-
- Mayilyan KR, Presanis JS, Arnold JN, Sim RB. Discrete MBL-MASP complexes show wide inter-individual variability in concentration: data from UK vs Armenian populations. Int J Immunopathol Pharmacol. 2006;19:567–80. - PubMed
-
- Bhakdi S, Tranum-Jensen J. Complement lysis: a hole is a hole. Immunol Today. 1991;12:318–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

