Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity
- PMID: 20002791
- PMCID: PMC2855792
- DOI: 10.1111/j.1365-2567.2009.03211.x
Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity
Abstract
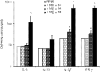
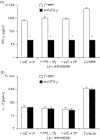
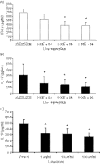
Both interferon-gamma-producing type 1 T helper (Th1)- and interleukin-17 (IL-17)-producing Th17 cells have been proposed to be involved in anti-fungal host defence. Although invasive aspergillosis is one of the most severe human fungal infections, little is known regarding the relative importance of the Th1 versus Th17 cellular immune pathways for the human anti-Aspergillus host defence. Using human peripheral blood mononuclear cells and a system consisting of monocyte-derived macrophages with lymphocytes, we found that Aspergillus fumigatus is a weak inducer of human IL-17 but induces a strong Th1 response. These data were validated by the very low IL-17 levels in bronchoalveolar lavage fluid and serum of patients with invasive aspergillosis. Surprisingly, live A. fumigatus reduced IL-17 production induced by mitogenic stimuli. This effect was mediated through the propensity of A. fumigatus to metabolize tryptophan and release kynurenine, which modulates the inflammatory response through inhibition of IL-17 production. In conclusion, A. fumigatus does not stimulate production of IL-17 and human host defence against aspergillosis may not rely on potent Th17 responses.
Figures

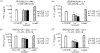

References
-
- Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Aspergillus Study Group. Medicine (Balt) 2000;79:250–60. - PubMed
-
- Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. - PubMed
-
- Stevens DA. Th1/Th2 in aspergillosis. Med Mycol. 2006;1:229–35. - PubMed
-
- Romani L. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr Opin Microbiol. 1999;2:363–7. - PubMed
-
- Chai LY, Netea MG, Vonk AG, Kullberg BJ. Fungal strategies for overcoming host innate immune response. Med Mycol. 2009;47:227–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

