Myostatin and follistatin expression in skeletal muscles of rats with chronic heart failure
- PMID: 20002838
- PMCID: PMC2812728
- DOI: 10.1111/j.1365-2613.2009.00683.x
Myostatin and follistatin expression in skeletal muscles of rats with chronic heart failure
Abstract
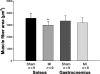
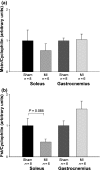
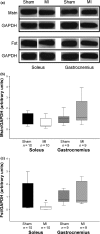
Skeletal muscle abnormalities can contribute to decreased exercise capacity in heart failure. Although muscle atrophy is a common alteration in heart failure, the mechanisms responsible for muscle mass reduction are not clear. Myostatin, a member of TGF-beta family (transforming growth factor), regulates muscle growth and mass. Several studies have shown a negative correlation between myostatin expression and muscle mass. The aim of this study was to evaluate myostatin expression in skeletal muscles of rats with heart failure. As myostatin gene expression can be modulated by follistatin, we also evaluated its expression. Heart failure was induced by myocardial infarction (MI, n = 10); results were compared to Sham-operated group (n = 10). Ventricular function was assessed by echocardiogram. Gene expression was analyzed by real-time PCR and protein levels by Western blotting in the soleus and gastrocnemius muscles; fibre trophism was evaluated by morphometric analysis. MI group presented heart failure evidence such as pleural effusion and right ventricular hypertrophy. Left ventricular dilation and dysfunction were observed in MI group. In the soleus muscle, cross-sectional area (P = 0.006) and follistatin protein levels (Sham 1.00 +/- 0.36; MI 0.18 +/- 0.06 arbitrary units; P = 0.03) were lower in MI and there was a trend for follistatin gene expression to be lower in MI group (P = 0.085). There was no change in myostatin expression between groups. In gastrocnemius, all MI group parameters were statistically similar to the Sham. In conclusion, our data show that during chronic heart failure, decreased skeletal muscle trophism is combined with unchanged myostatin and reduced follistatin expression.
Figures

Similar articles
-
Myocardial myostatin in spontaneously hypertensive rats with heart failure.Int J Cardiol. 2016 Jul 15;215:384-7. doi: 10.1016/j.ijcard.2016.04.101. Epub 2016 Apr 15. Int J Cardiol. 2016. PMID: 27128567
-
Heart failure-induced skeletal myopathy in spontaneously hypertensive rats.Int J Cardiol. 2013 Aug 10;167(3):698-703. doi: 10.1016/j.ijcard.2012.03.063. Epub 2012 Mar 30. Int J Cardiol. 2013. PMID: 22464481
-
Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure.Circulation. 2010 Jan 26;121(3):419-25. doi: 10.1161/CIRCULATIONAHA.109.882068. Epub 2010 Jan 11. Circulation. 2010. PMID: 20065166 Free PMC article.
-
Myostatin from the heart: local and systemic actions in cardiac failure and muscle wasting.Am J Physiol Heart Circ Physiol. 2011 Jun;300(6):H1973-82. doi: 10.1152/ajpheart.00200.2011. Epub 2011 Mar 18. Am J Physiol Heart Circ Physiol. 2011. PMID: 21421824 Free PMC article. Review.
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
Cited by
-
Association between Functional Variables and Heart Failure after Myocardial Infarction in Rats.Arq Bras Cardiol. 2016 Feb;106(2):105-12. doi: 10.5935/abc.20160015. Epub 2016 Jan 26. Arq Bras Cardiol. 2016. PMID: 26815462 Free PMC article.
-
Activins and Inhibins: Roles in Development, Physiology, and Disease.Cold Spring Harb Perspect Biol. 2016 Jul 1;8(7):a021881. doi: 10.1101/cshperspect.a021881. Cold Spring Harb Perspect Biol. 2016. PMID: 27328872 Free PMC article. Review.
-
Gut microbiota in sarcopenia and heart failure.J Cardiovasc Aging. 2022 Jul;2(3):35. doi: 10.20517/jca.2022.07. Epub 2022 Jul 5. J Cardiovasc Aging. 2022. PMID: 35891702 Free PMC article.
-
Beneficial Effects of Physical Exercise on Functional Capacity and Skeletal Muscle Oxidative Stress in Rats with Aortic Stenosis-Induced Heart Failure.Oxid Med Cell Longev. 2016;2016:8695716. doi: 10.1155/2016/8695716. Epub 2016 Jan 20. Oxid Med Cell Longev. 2016. PMID: 26904168 Free PMC article.
-
Skeletal muscle aging: influence of oxidative stress and physical exercise.Oncotarget. 2017 Mar 21;8(12):20428-20440. doi: 10.18632/oncotarget.14670. Oncotarget. 2017. PMID: 28099900 Free PMC article. Review.
References
-
- Acharyya S, Gutttridge DC. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin. Cancer Res. 2007;13:1356–1361. - PubMed
-
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. - PubMed
-
- Anker SD, Coats AJS. Cardiac cachexia A syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115:836–847. - PubMed
-
- Baumann AP, Ibebunjo C, Grasser WA, Paralkar VM. Myostatin expression in age and denervation-induced skeletal muscle atrophy. J. Musculoskelet. Neuronal. Interact. 2003;3:8–16. - PubMed
-
- Bing OHL, Brooks WW, Robinson KG, et al. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J. Mol. Cell. Cardiol. 1995;27:383–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

