Transforming growth factor-beta inhibits aromatase gene transcription in human trophoblast cells via the Smad2 signaling pathway
- PMID: 20003198
- PMCID: PMC2797513
- DOI: 10.1186/1477-7827-7-146
Transforming growth factor-beta inhibits aromatase gene transcription in human trophoblast cells via the Smad2 signaling pathway
Abstract
Background: Transforming growth factor-beta (TGF-beta) is known to exert multiple regulatory functions in the human placenta, including inhibition of estrodial production. We have previously reported that TGF-beta1 decreased aromatase mRNA levels in human trophoblast cells. The objective of this study was to investigate the molecular mechanisms underlying the regulatory effect of TGF-beta1 on aromatase expression.
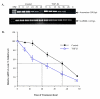
Methods: To determine if TGF-beta regulates aromatase gene transcription, several reporter constructs containing different lengths of the placental specific promoter of the human aromatase gene were generated. JEG-3 cells were transiently transfected with a promoter construct and treated with or without TGF-beta1. The promoter activity was measured by luciferase assays. To examine the downstream signaling molecule mediating the effect of TGF-beta on aromatase transcription, cells were transiently transfected with dominant negative mutants of TGF-beta type II (TbetaRII) and type I receptor (ALK5) receptors before TGF-beta treatment. Smad2 activation was assessed by measuring phophorylated Smad2 protein levels in cytosolic and nuclear fractions. Smad2 expression was silenced using a siRNA expression construct. Finally, aromatase mRNA half-life was determined by treating cells with actinomycin D together with TGF-beta1 and measuring aromatase mRNA levels at various time points after treatment.
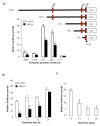
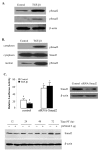
Results and discussion: TGF-beta1 inhibited the aromatase promoter activity in a time- and dose-dependent manner. Deletion analysis suggests that the TGF-beta1 response element resides between -422 and -117 nucleotides upstream from the transcription start site where a Smad binding element was found. The inhibitory effect of TGF-beta1 was blocked by dominant negative mutants of TbetaRII and ALK5. TGF-beta1 treatment induced Smad2 phosphorylation and translocation into the nucleus. On the other hand, knockdown of Smad2 expression reversed the inhibitory effect of TGF-beta1 on aroamtase transcription. Furthermore, TGF-beta1 accelerated the degradation of aromatase mRNA.
Conclusion: Our results demonstrate that TGF-beta1 exerts regulatory effects on aromatase gene at both transcriptional and post-transcriptional levels. The transcriptional regulation of aromatase gene by TGF-beta1 is mediated by the canonical TGF-beta pathway involving TbetaRII, ALK5 and Smad2. These findings further support the role of TGF-beta1 in regulating human placental functions and pregnancy.
Figures


References
-
- Peng C. The TGF-beta superfamily and its roles in the human ovary and placenta. J Obstet Gynaecol Can. 2003;25(10):834–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

