Identifying sexual differentiation genes that affect Drosophila life span
- PMID: 20003237
- PMCID: PMC2803781
- DOI: 10.1186/1471-2318-9-56
Identifying sexual differentiation genes that affect Drosophila life span
Abstract
Background: Sexual differentiation often has significant effects on life span and aging phenotypes. For example, males and females of several species have different life spans, and genetic and environmental manipulations that affect life span often have different magnitude of effect in males versus females. Moreover, the presence of a differentiated germ-line has been shown to affect life span in several species, including Drosophila and C. elegans.
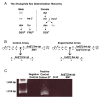
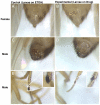
Methods: Experiments were conducted to determine how alterations in sexual differentiation gene activity might affect the life span of Drosophila melanogaster. Drosophila females heterozygous for the tudor[1] mutation produce normal offspring, while their homozygous sisters produce offspring that lack a germ line. To identify additional sexual differentiation genes that might affect life span, the conditional transgenic system Geneswitch was employed, whereby feeding adult flies or developing larvae the drug RU486 causes the over-expression of selected UAS-transgenes.
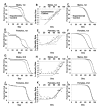
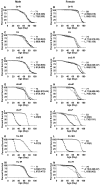
Results: In this study germ-line ablation caused by the maternal tudor[1] mutation was examined in a long-lived genetic background, and was found to increase life span in males but not in females, consistent with previous reports. Fitting the data to a Gompertz-Makeham model indicated that the maternal tudor[1] mutation increases the life span of male progeny by decreasing age-independent mortality. The Geneswitch system was used to screen through several UAS-type and EP-type P element mutations in genes that regulate sexual differentiation, to determine if additional sex-specific effects on life span would be obtained. Conditional over-expression of transformer female isoform (traF) during development produced male adults with inhibited sexual differentiation, however this caused no significant change in life span. Over-expression of doublesex female isoform (dsxF) during development was lethal to males, and produced a limited number of female escapers, whereas over-expression of dsxF specifically in adults greatly reduced both male and female life span. Similarly, over-expression of fruitless male isoform A (fru-MA) during development was lethal to both males and females, whereas over-expression of fru-MA in adults greatly reduced both male and female life span.
Conclusion: Manipulation of sexual differentiation gene expression specifically in the adult, after morphological sexual differentiation is complete, was still able to affect life span. In addition, by manipulating gene expression during development, it was possible to significantly alter morphological sexual differentiation without a significant effect on adult life span. The data demonstrate that manipulation of sexual differentiation pathway genes either during development or in adults can affect adult life span.
Figures
Similar articles
-
A screen of apoptosis and senescence regulatory genes for life span effects when over-expressed in Drosophila.Aging (Albany NY). 2009 Jan 30;1(2):191-211. doi: 10.18632/aging.100018. Aging (Albany NY). 2009. PMID: 20157509 Free PMC article.
-
Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy.Aging (Albany NY). 2009 Oct 27;1(11):903-36. doi: 10.18632/aging.100099. Aging (Albany NY). 2009. PMID: 20157574 Free PMC article.
-
Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless.Genetics. 2001 Aug;158(4):1569-95. doi: 10.1093/genetics/158.4.1569. Genetics. 2001. PMID: 11514448 Free PMC article.
-
Neurogenetics of courtship and mating in Drosophila.Adv Genet. 2008;62:67-184. doi: 10.1016/S0065-2660(08)00603-2. Adv Genet. 2008. PMID: 19010254 Review.
-
Genetic control of germline sexual dimorphism in Drosophila.Int Rev Cytol. 2002;219:1-60. doi: 10.1016/s0074-7696(02)19010-3. Int Rev Cytol. 2002. PMID: 12211627 Review.
Cited by
-
Dying occurs as a defined molecular progression in Drosophila rather than as nonspecific physiological collapse.iScience. 2025 Jul 14;28(8):113115. doi: 10.1016/j.isci.2025.113115. eCollection 2025 Aug 15. iScience. 2025. PMID: 40755455 Free PMC article.
-
Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster.Aging (Albany NY). 2012 Nov;4(11):768-89. doi: 10.18632/aging.100499. Aging (Albany NY). 2012. PMID: 23211361 Free PMC article.
-
CRISPR/Cas9 mediated sex-ratio distortion by sex specific gene editing in Aedes aegypti.Saudi J Biol Sci. 2022 Apr;29(4):3015-3022. doi: 10.1016/j.sjbs.2022.01.034. Epub 2022 Jan 25. Saudi J Biol Sci. 2022. PMID: 35531165 Free PMC article.
-
Sex differences in the response to oxidative and proteolytic stress.Redox Biol. 2020 Apr;31:101488. doi: 10.1016/j.redox.2020.101488. Epub 2020 Mar 9. Redox Biol. 2020. PMID: 32201219 Free PMC article. Review.
-
Mifepristone/RU486 acts in Drosophila melanogaster females to counteract the life span-shortening and pro-inflammatory effects of male Sex Peptide.Biogerontology. 2017 Jun;18(3):413-427. doi: 10.1007/s10522-017-9703-y. Epub 2017 Apr 27. Biogerontology. 2017. PMID: 28451923 Free PMC article.
References
-
- Kirkwood TBL. In: Genetic effects on aging II. Harrison DE, editor. Caldwell, NJ: Telford press.; 1990. The disposable soma theory of aging.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

