Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways
- PMID: 20003259
- PMCID: PMC2804605
- DOI: 10.1186/1476-4598-8-118
Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways
Abstract
Background: Osteosarcoma (OS) is the most common primary bone tumour in children and young adults. Despite improved prognosis, metastatic or relapsed OS remains largely incurable and no significant improvement has been observed in the last 20 years. Therefore, the search for alternative agents in OS is mandatory.
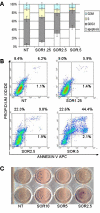
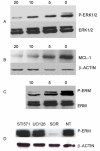
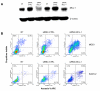
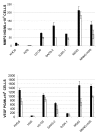
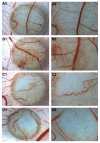
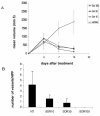
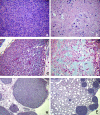
Results: We investigated phospho-ERK 1/2, MCL-1, and phospho-Ezrin/Radixin/Moesin (P-ERM) as potential therapeutic targets in OS. Activation of these pathways was shown by immunohistochemistry in about 70% of cases and in all OS cell lines analyzed. Mutational analysis revealed no activating mutations in KRAS whereas BRAF gene was found to be mutated in 4/30 OS samples from patients. Based on these results we tested the multi-kinase inhibitor sorafenib (BAY 43-9006) in preclinical models of OS. Sorafenib inhibited OS cell line proliferation, induced apoptosis and downregulated P-ERK1/2, MCL-1, and P-ERM in a dose-dependent manner. The dephosphorylation of ERM was not due to ERK inhibition. The downregulation of MCL-1 led to an increase in apoptosis in OS cell lines. In chick embryo chorioallantoic membranes, OS supernatants induced angiogenesis, which was blocked by sorafenib and it was also shown that sorafenib reduced VEGF and MMP2 production. In addition, sorafenib treatment dramatically reduced tumour volume of OS xenografts and lung metastasis in SCID mice.
Conclusion: In conclusion, ERK1/2, MCL-1 and ERM pathways are shown to be active in OS. Sorafenib is able to inhibit their signal transduction, both in vitro and in vivo, displaying anti-tumoural activity, anti-angiogenic effects, and reducing metastatic colony formation in lungs. These data support the testing of sorafenib as a potential therapeutic option in metastatic or relapsed OS patients unresponsive to standard treatments.
Figures

References
-
- Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, Müller C, Tienghi A, Wiebe T, Comandone A, Böhling T, Del Prever AB, Brosjö O, Bacci G, Saeter G. Italian and Scandinavian Sarcoma Groups. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–52. doi: 10.1200/JCO.2004.00.5785. - DOI - PubMed
-
- Ozaki T, Flege S, Kevric M, Lindner N, Maas R, Delling G, Schwarz R, von Hochstetter AR, Salzer-Kuntschik M, Berdel WE, Jürgens H, Exner GU, Reichardt P, Mayer-Steinacker R, Ewerbeck V, Kotz R, Winkelmann W, Bielack SS. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21:334–41. doi: 10.1200/JCO.2003.01.142. - DOI - PubMed
-
- Serra M, Pasello M, Manara MC, Scotlandi K, Ferrari S, Bertoni F, Mercuri M, Alvegard TA, Picci P, Bacci G, Smeland S. May P-glycoprotein status be used to stratify high-grade osteosarcoma patients? Results from the Italian/Scandinavian Sarcoma Group 1 treatment protocol. Int J Oncol. 2006;29:1459–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

