Pharmacokinetics and lung delivery of PDDS-aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia
- PMID: 20003269
- PMCID: PMC2811890
- DOI: 10.1186/cc8206
Pharmacokinetics and lung delivery of PDDS-aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia
Abstract
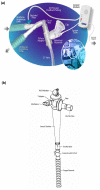
Introduction: Aminoglycosides aerosolization might achieve better diffusion into the alveolar compartment than intravenous use. The objective of this multicenter study was to evaluate aerosol-delivered amikacin penetration into the alveolar epithelial lining fluid (ELF) using a new vibrating mesh nebulizer (Pulmonary Drug Delivery System (PDDS), Nektar Therapeutics), which delivers high doses to the lungs.
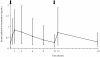
Methods: Nebulized amikacin (400 mg bid) was delivered to the lungs of 28 mechanically ventilated patients with Gram-negative VAP for 7-14 days, adjunctive to intravenous therapy. On treatment day 3, 30 minutes after completing aerosol delivery, all the patients underwent bronchoalveolar lavage in the infection-involved area and the ELF amikacin concentration was determined. The same day, urine and serum amikacin concentrations were determined at different time points.
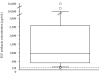
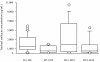
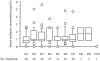
Results: Median (range) ELF amikacin and maximum serum amikacin concentrations were 976.1 (135.7-16127.6) and 0.9 (0.62-1.73) microg/mL, respectively. The median total amount of amikacin excreted in urine during the first and second 12-hour collection on day 3 were 19 (12.21-28) and 21.2 (14.1-29.98) microg, respectively. During the study period, daily through amikacin measurements were below the level of nephrotoxicity. Sixty-four unexpected adverse events were reported, among which 2 were deemed possibly due to nebulized amikacin: one episode of worsening renal failure, and one episode of bronchospasm.
Conclusions: PDDS delivery of aerosolized amikacin achieved very high aminoglycoside concentrations in ELF from radiography-controlled infection-involved zones, while maintaining safe serum amikacin concentrations. The ELF concentrations always exceeded the amikacin minimum inhibitory concentrations for Gram-negative microorganisms usually responsible for these pneumonias. The clinical impact of amikacin delivery with this system remains to be determined.
Trial registration: ClinicalTrials.gov Identifier: NCT01021436.
Figures
References
-
- Boselli E, Breilh D, Djabarouti S, Guillaume C, Rimmele T, Gordien JB, Xuereb F, Saux MC, Allaouchiche B. Reliability of mini-bronchoalveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med. 2007;33:1519–1523. doi: 10.1007/s00134-007-0688-x. - DOI - PubMed
-
- Goldstein I, Wallet F, Robert J, Becquemin MH, Marquette CH, Rouby JJ. Lung tissue concentrations of nebulized amikacin during mechanical ventilation in piglets with healthy lungs. Am J Respir Crit Care Med. 2002;165:171–175. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

