RANKL inhibition by osteoprotegerin prevents bone loss without affecting local or systemic inflammation parameters in two rat arthritis models: comparison with anti-TNFalpha or anti-IL-1 therapies
- PMID: 20003323
- PMCID: PMC3003514
- DOI: 10.1186/ar2879
RANKL inhibition by osteoprotegerin prevents bone loss without affecting local or systemic inflammation parameters in two rat arthritis models: comparison with anti-TNFalpha or anti-IL-1 therapies
Abstract
Introduction: Rat adjuvant-induced arthritis (AIA) and collagen-induced arthritis (CIA) feature bone loss and systemic increases in TNFalpha, IL-1beta, and receptor activator of NF-kappaB ligand (RANKL). Anti-IL-1 or anti-TNFalpha therapies consistently reduce inflammation in these models, but systemic bone loss often persists. RANKL inhibition consistently prevents bone loss in both models without reducing joint inflammation. Effects of these therapies on systemic markers of bone turnover and inflammation have not been directly compared.
Methods: Lewis rats with established AIA or CIA were treated for 10 days (from day 4 post onset) with either PBS (Veh), TNFalpha inhibitor (pegsunercept), IL-1 inhibitor (anakinra), or RANKL inhibitor (osteoprotegerin (OPG)-Fc). Local inflammation was evaluated by monitoring hind paw swelling. Bone mineral density (BMD) of paws and lumbar vertebrae was assessed by dual X-ray absorptiometry. Markers and mediators of bone resorption (RANKL, tartrate-resistant acid phosphatase 5b (TRACP 5B)) and inflammation (prostaglandin E2 (PGE2), acute-phase protein alpha-1-acid glycoprotein (alpha1AGP), multiple cytokines) were measured in serum (day 14 post onset).
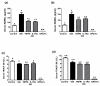
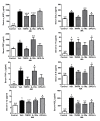
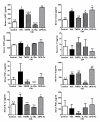
Results: Arthritis progression significantly increased paw swelling and ankle and vertebral BMD loss. Anti-TNFalpha reduced paw swelling in both models, and reduced ankle BMD loss in AIA rats. Anti-IL-1 decreased paw swelling in CIA rats, and reduced ankle BMD loss in both models. Anti-TNFalpha and anti-IL-1 failed to prevent vertebral BMD loss in either model. OPG-Fc reduced BMD loss in ankles and vertebrae in both models, but had no effect on paw swelling. Serum RANKL was elevated in AIA-Veh and CIA-Veh rats. While antiTNFalpha and anti-IL-1 partially normalized serum RANKL without any changes in serum TRACP 5B, OPG-Fc treatment reduced serum TRACP 5B by over 90% in both CIA and AIA rats. CIA-Veh and AIA-Veh rats had increased serum alpha1AGP, IL-1beta, IL-8 and chemokine (C-C motif) ligand 2 (CCL2), and AIA-Veh rats also had significantly greater serum PGE2, TNFalpha and IL-17. Anti-TNFalpha reduced systemic alpha1AGP, CCL2 and PGE2 in AIA rats, while anti-IL-1 decreased systemic alpha1AGP, IL-8 and PGE2. In contrast, RANKL inhibition by OPG-Fc did not lessen systemic cytokine levels in either model.
Conclusions: Anti-TNFalpha or anti-IL-1 therapy inhibited parameters of local and systemic inflammation, and partially reduced local but not systemic bone loss in AIA and CIA rats. RANKL inhibition prevented local and systemic bone loss without significantly inhibiting local or systemic inflammatory parameters.
Figures




Similar articles
-
RANKL is a marker and mediator of local and systemic bone loss in two rat models of inflammatory arthritis.J Bone Miner Res. 2005 Oct;20(10):1756-65. doi: 10.1359/JBMR.050601. Epub 2005 Jun 6. J Bone Miner Res. 2005. PMID: 16160733
-
Additive bone-protective effects of anabolic treatment when used in conjunction with RANKL and tumor necrosis factor inhibition in two rat arthritis models.Arthritis Rheum. 2005 May;52(5):1604-11. doi: 10.1002/art.21021. Arthritis Rheum. 2005. PMID: 15880601
-
Anti-rheumatoid arthritis effects of iridoid glucosides from Lamiophlomis rotata (Benth.) kudo on adjuvant-induced arthritis in rats by OPG/RANKL/NF-κB signaling pathways.J Ethnopharmacol. 2021 Feb 10;266:113402. doi: 10.1016/j.jep.2020.113402. Epub 2020 Sep 24. J Ethnopharmacol. 2021. PMID: 32980481
-
The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models.Arthritis Res Ther. 2005;7(1):29-37. doi: 10.1186/ar1478. Epub 2004 Nov 30. Arthritis Res Ther. 2005. PMID: 15642151 Free PMC article. Review.
-
Utility of animal models for identification of potential therapeutics for rheumatoid arthritis.Ann Rheum Dis. 2008 Nov;67(11):1505-15. doi: 10.1136/ard.2007.076430. Epub 2007 Nov 29. Ann Rheum Dis. 2008. PMID: 18055474 Review.
Cited by
-
The Role of Oral Pathobionts in Dysbiosis during Periodontitis Development.J Dent Res. 2014 Jun;93(6):539-46. doi: 10.1177/0022034514528212. Epub 2014 Mar 19. J Dent Res. 2014. PMID: 24646638 Free PMC article. Review.
-
Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis.Arthritis Res Ther. 2011 Jul 28;13(4):235. doi: 10.1186/ar3380. Arthritis Res Ther. 2011. PMID: 21861862 Free PMC article. Review.
-
Effect of sclerostin-neutralising antibody on periarticular and systemic bone in a murine model of rheumatoid arthritis: a microCT study.Arthritis Res Ther. 2013;15(5):R125. doi: 10.1186/ar4305. Arthritis Res Ther. 2013. PMID: 24432364 Free PMC article.
-
Inhibition of osteoclastogenesis by RNA interference targeting RANK.BMC Musculoskelet Disord. 2012 Aug 22;13:154. doi: 10.1186/1471-2474-13-154. BMC Musculoskelet Disord. 2012. PMID: 22913338 Free PMC article.
-
Mechanisms of Systemic Osteoporosis in Rheumatoid Arthritis.Int J Mol Sci. 2022 Aug 5;23(15):8740. doi: 10.3390/ijms23158740. Int J Mol Sci. 2022. PMID: 35955873 Free PMC article. Review.
References
-
- Brennan FM, Field M, Chu CQ, Feldmann M, Maini RN. Cytokine expression in rheumatoid arthritis. Br J Rheumatol. 1991;30(Suppl 1):76–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

