Role of p53 mutation in the effect of boron neutron capture therapy on oral squamous cell carcinoma
- PMID: 20003329
- PMCID: PMC2803486
- DOI: 10.1186/1748-717X-4-63
Role of p53 mutation in the effect of boron neutron capture therapy on oral squamous cell carcinoma
Abstract
Background: Boron neutron capture therapy (BNCT) is a selective radiotherapy, being effective for the treatment of even advanced malignancies in head and neck regions as well as brain tumors and skin melanomas. To clarify the role of p53 gene, the effect of BNCT on oral squamous cell carcinoma (SCC) cells showing either wild- (SAS/neo) or mutant-type (SAS/mp53) p53 was examined.
Methods: Cells were exposed to neutron beams in the presence of boronophenylalanine (BPA) at Kyoto University Research Reactor. Treated cells were monitored for modulations in colony formation, proliferation, cell cycle, and expression of cell cycle-associated proteins.
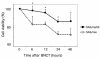
Results: When SAS/neo and SAS/mp53 cells were subjected to BNCT, more suppressive effects on colony formation and cell viability were observed in SAS/neo compared with SAS/mp53 cells. Cell cycle arrest at the G1 checkpoint was observed in SAS/neo, but not in SAS/mp53. Apoptotic cells increased from 6 h after BNCT in SAS/neo and 48 h in SAS/mp53 cells. The expression of p21 was induced in SAS/neo only, but G2 arrest-associated proteins including Wee1, cdc2, and cyclin B1 were altered in both cell lines.
Conclusion: These results indicate that oral SCC cells with mutant-type are more resistant to BNCT than those with wild-type p53, and that the lack of G1 arrest and related apoptosis may contribute to the resistance. At a physical dose affecting the cell cycle, BNCT inhibits oral SCC cells in p53-dependent and -independent manners.
Figures


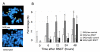


References
-
- Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. - PubMed
-
- Offer H, Zurer I, Banfalvi G, Reha'k M, Falcovitz A, Milyavsky M, Goldfinger N, Rotter V. p53 modulates base excision repair activity in a cell cycle-specific manner after genotoxic stress. Cancer Res. 2001;61:88–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

