Intraventricular infusion of hyperosmolar dextran induces hydrocephalus: a novel animal model of hydrocephalus
- PMID: 20003330
- PMCID: PMC2801660
- DOI: 10.1186/1743-8454-6-16
Intraventricular infusion of hyperosmolar dextran induces hydrocephalus: a novel animal model of hydrocephalus
Abstract
Background: Popular circulation theory of hydrocephalus assumes that the brain is impermeable to cerebrospinal fluid (CSF), and is therefore incapable of absorbing the CSF accumulating within the ventricles. However, the brain parenchyma is permeable to water due to the presence of specific ion channels as well as aquaporin channels. Thus, the movement of water into and out of the ventricles may be determined by the osmotic load of the CSF. If osmotic load determines the aqueous content of CSF in this manner, it is reasonable to hypothesize that hydrocephalus may be precipitated by pathologies and/or insults that produce sustained elevations of osmotic content within the ventricles.
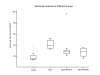
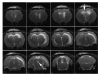
Methods: We investigated this hypothesis by manipulating the osmotic content of CSF and assaying the development of hydrocephalus in the rat brain. This was achieved by continuously infusing artificial CSF (negative control; group I), fibroblast growth factor (FGF2) solution (positive control; group II) and hyperosmotic dextran solutions (10 KD and 40 KD as experimental solutions: groups III and IV) for 12 days at 0.5 muL/h. The osmolality of the fluid infused was 307, 664, 337 and 328 mOsm/L in Groups I, II, III and IV, respectively. Magnetic resonance imaging (MRI) was used to evaluate the ventricular volumes. Analysis of variance (ANOVA) with pairwise group comparisons was done to assess the differences in ventricular volumes among the four groups.
Results: Group I had no hydrocephalus. Group II, group III and group IV animals exhibited significant enlargement of the ventricles (hydrocephalus) compared to group I. There was no statistically significant difference in the size of the ventricles between groups II, III and IV. None of the animals with hydrocephalus had obstruction of the aqueduct or other parts of CSF pathways on MRI.
Conclusion: Infusing hyperosmolar solutions of dextran, or FGF into the ventricles chronically, resulted in ventricular enlargement. These solutions increase the osmotic load in the ventricles. Water influx (through the choroid plexus CSF secretion and/or through the brain) into the ventricles to normalize this osmotic gradient results in hydrocephalus. We need to revise the popular theory of how fluid accumulates in the ventricles at least in some forms of hydrocephalus.
Figures

Similar articles
-
Increased CSF osmolarity reversibly induces hydrocephalus in the normal rat brain.Fluids Barriers CNS. 2012 Jul 11;9(1):13. doi: 10.1186/2045-8118-9-13. Fluids Barriers CNS. 2012. PMID: 22784705 Free PMC article.
-
New experimental model of acute aqueductal blockage in cats: effects on cerebrospinal fluid pressure and the size of brain ventricles.Neuroscience. 2009 Feb 18;158(4):1397-405. doi: 10.1016/j.neuroscience.2008.11.041. Epub 2008 Dec 7. Neuroscience. 2009. PMID: 19111908
-
Post-injury ventricular enlargement associates with iron in choroid plexus but not with seizure susceptibility nor lesion atrophy-6-month MRI follow-up after experimental traumatic brain injury.Brain Struct Funct. 2022 Jan;227(1):145-158. doi: 10.1007/s00429-021-02395-5. Epub 2021 Nov 10. Brain Struct Funct. 2022. PMID: 34757444 Free PMC article.
-
The normal and pathological physiology of brain water.Adv Tech Stand Neurosurg. 1997;23:47-142. doi: 10.1007/978-3-7091-6549-2_2. Adv Tech Stand Neurosurg. 1997. PMID: 9075471 Review.
-
New concepts in the pathogenesis of hydrocephalus.Transl Pediatr. 2014 Jul;3(3):185-94. doi: 10.3978/j.issn.2224-4336.2014.07.02. Transl Pediatr. 2014. PMID: 26835336 Free PMC article. Review.
Cited by
-
Posthemispherectomy hydrocephalus: results of a comprehensive, multiinstitutional review.Epilepsia. 2013 Feb;54(2):383-9. doi: 10.1111/epi.12010. Epub 2012 Oct 25. Epilepsia. 2013. PMID: 23106378 Free PMC article.
-
Increased CSF osmolarity reversibly induces hydrocephalus in the normal rat brain.Fluids Barriers CNS. 2012 Jul 11;9(1):13. doi: 10.1186/2045-8118-9-13. Fluids Barriers CNS. 2012. PMID: 22784705 Free PMC article.
-
Development of an acute obstructive hydrocephalus model in rats using N-butyl cyanoacrylate.Childs Nerv Syst. 2011 Jun;27(6):903-10. doi: 10.1007/s00381-011-1398-9. Epub 2011 Feb 1. Childs Nerv Syst. 2011. PMID: 21286731
-
Development of Microfabricated Magnetic Actuators for Removing Cellular Occlusion.J Micromech Microeng. 2011 May;21(5):54006. doi: 10.1088/0960-1317/21/5/054006. J Micromech Microeng. 2011. PMID: 21886945 Free PMC article.
-
Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage.Transl Stroke Res. 2012 Jul;3(Suppl 1):25-38. doi: 10.1007/s12975-012-0182-9. Transl Stroke Res. 2012. PMID: 23976902 Free PMC article.
References
-
- Sainte-Rose C. In: Neurological Surgery. 4. Youmans JR, editor. Philadelphia: WB Saunders; 1996. Hydrocephalus in childhood; pp. 890–922.
-
- Rekate HL. In: Neurological Surgery. 5. Youmans JR, editor. Philadelphia: WB Saunders; 2004. Hydrocephalus in children; pp. 3387–3404.
-
- McAllister JP, II, Chovan P. In: Neurosurg Clin N America, Neurosurgery of the Neonate. Frim DM, Madsen JR, editor. Philadelphia: W.B. Saunders; 1998. Neonatal hydrocephalus: mechanisms and consequences; pp. 73–93. - PubMed
-
- Del Bigio MR, McAllister JP. , II. In: Pediatric Neurosurgery. Choux M, DiRocco CE, Hockley AD, Walker ML, editor. London: Churchill Livingstone; 1999. Pathophysiology of hydrocephalus; pp. 217–236.
LinkOut - more resources
Full Text Sources

