Identification of a biomarker panel using a multiplex proximity ligation assay improves accuracy of pancreatic cancer diagnosis
- PMID: 20003342
- PMCID: PMC2796647
- DOI: 10.1186/1479-5876-7-105
Identification of a biomarker panel using a multiplex proximity ligation assay improves accuracy of pancreatic cancer diagnosis
Abstract
Background: Pancreatic cancer continues to prove difficult to clinically diagnose. Multiple simultaneous measurements of plasma biomarkers can increase sensitivity and selectivity of diagnosis. Proximity ligation assay (PLA) is a highly sensitive technique for multiplex detection of biomarkers in plasma with little or no interfering background signal.
Methods: We examined the plasma levels of 21 biomarkers in a clinically defined cohort of 52 locally advanced (Stage II/III) pancreatic ductal adenocarcinoma cases and 43 age-matched controls using a multiplex proximity ligation assay. The optimal biomarker panel for diagnosis was computed using a combination of the PAM algorithm and logistic regression modeling. Biomarkers that were significantly prognostic for survival in combination were determined using univariate and multivariate Cox survival models.
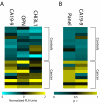
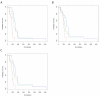
Results: Three markers, CA19-9, OPN and CHI3L1, measured in multiplex were found to have superior sensitivity for pancreatic cancer vs. CA19-9 alone (93% vs. 80%). In addition, we identified two markers, CEA and CA125, that when measured simultaneously have prognostic significance for survival for this clinical stage of pancreatic cancer (p < 0.003).
Conclusions: A multiplex panel assaying CA19-9, OPN and CHI3L1 in plasma improves accuracy of pancreatic cancer diagnosis. A panel assaying CEA and CA125 in plasma can predict survival for this clinical cohort of pancreatic cancer patients.
Figures

References
-
- Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, (Eds) SEER Cancer Statistics Review, 1975-2005. Bethesda, MD: National Cancer Institute; 2008.
-
- Frebourg T, Bercoff E, Manchon N, Senant J, Basuyau JP, Breton P, Janvresse A, Brunelle P, Bourreille J. The evaluation of CA 19-9 antigen level in the early detection of pancreatic cancer. A prospective study of 866 patients. Cancer. 1988;62:2287–2290. doi: 10.1002/1097-0142(19881201)62:11<2287::AID-CNCR2820621103>3.0.CO;2-H. - DOI - PubMed
-
- Magnani JL, Steplewski Z, Koprowski H, Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983;43:5489–5492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

