Cost-utility analysis of infliximab and adalimumab for refractory ulcerative colitis
- PMID: 20003364
- PMCID: PMC2797497
- DOI: 10.1186/1478-7547-7-20
Cost-utility analysis of infliximab and adalimumab for refractory ulcerative colitis
Abstract
Objective: To evaluate cost-utility of infliximab and adalimumab for the treatment of moderate-to-severe ulcerative colitis (UC) refractory to conventional therapies in Canada.
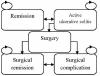
Methods: A Markov model was constructed to evaluate incremental cost-utility ratios (ICUR) of 5 mg/kg and 10 mg/kg infliximab and adalimumab therapies compared to 'usual care' in treating a hypothetical cohort of patients (aged 40 years and weighing 80 kg) over a five-year time horizon from the perspective of a publicly-funded health care system. Clinical parameters were derived from the Active Ulcerative Colitis Trials 1 and 2. Costs were obtained through provincial drug benefit plans. ICUR was the main outcome measure and both deterministic and probabilistic sensitivity analyses were conducted.
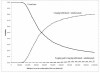
Results: Compared to the strategy A ('usual care') in the base case analysis, the ICURs were CA$358,088/QALY for the strategy B ('5 mg/kg infliximab + adalimumab') and CA$575,540/QALY for the strategy C ('5 mg/kg and 10 mg/kg infliximab + adalimumab'). The results were sensitive to: the remission rates maintained in responders to 'usual care' and to 5 mg/kg infliximab, the rate of remission induced by adalimumab in non-responders to 5 mg/kg infliximab, early surgery rate, and utility values. When the willingness to pay (WTP) was less than CA$150,000/QALY, the probability of 'usual care' being the optimal strategy was 1.0. The probability of strategy B being optimal was 0.5 when the WTP approximated CA$400,000/QALY.
Conclusions: The ICURs of anti-TNF-alpha drugs were not satisfactory in treating patients with moderate-to-severe refractory UC. Future research could be aimed at the long-term clinical benefits of these drugs, especially adalimumab for patients intolerant or unresponsive to infliximab treatment.
Figures
Similar articles
-
Cost-Utility Analysis of Infliximab with Standard Care versus Standard Care Alone for Induction and Maintenance Treatment of Patients with Ulcerative Colitis in Poland.Pharmacotherapy. 2016 May;36(5):472-81. doi: 10.1002/phar.1742. Epub 2016 May 2. Pharmacotherapy. 2016. PMID: 27007213
-
Canadian cost-utility analysis of initiation and maintenance treatment with anti-TNF-α drugs for refractory Crohn's disease.J Crohns Colitis. 2012 Feb;6(1):77-85. doi: 10.1016/j.crohns.2011.07.007. Epub 2011 Sep 9. J Crohns Colitis. 2012. PMID: 22261531
-
Cost-effectiveness analysis of infliximab, adalimumab, golimumab and vedolizumab for moderate to severe ulcerative colitis in Spain.Expert Rev Pharmacoecon Outcomes Res. 2018 Jun;18(3):321-329. doi: 10.1080/14737167.2018.1411193. Epub 2017 Dec 2. Expert Rev Pharmacoecon Outcomes Res. 2018. PMID: 29192530
-
A Systematic Review of the Cost-Effectiveness of Biologics for Ulcerative Colitis.Pharmacoeconomics. 2018 Apr;36(4):419-434. doi: 10.1007/s40273-017-0601-6. Pharmacoeconomics. 2018. PMID: 29260508 Free PMC article.
-
A review of infliximab use in ulcerative colitis.Clin Ther. 2008 Feb;30(2):223-30. doi: 10.1016/j.clinthera.2008.02.014. Clin Ther. 2008. PMID: 18343261 Review.
Cited by
-
Identification of the Most Cost-effective Position of Vedolizumab Among the Available Biologic Drugs for the Treatment of Ulcerative Colitis.J Crohns Colitis. 2020 Jun 19;14(5):575-587. doi: 10.1093/ecco-jcc/jjz212. J Crohns Colitis. 2020. PMID: 31901085 Free PMC article.
-
Cost-effectiveness of vedolizumab compared with conventional therapy for ulcerative colitis patients in the UK.Clinicoecon Outcomes Res. 2017 Oct 16;9:641-652. doi: 10.2147/CEOR.S135609. eCollection 2017. Clinicoecon Outcomes Res. 2017. PMID: 29081667 Free PMC article.
-
A Systematic Review of the Cost-Effectiveness of Biologics for the Treatment of Inflammatory Bowel Diseases.PLoS One. 2015 Dec 16;10(12):e0145087. doi: 10.1371/journal.pone.0145087. eCollection 2015. PLoS One. 2015. PMID: 26675292 Free PMC article.
-
Cost-effectiveness analysis of infliximab, adalimumab, golimumab, vedolizumab and tofacitinib for moderate to severe ulcerative colitis in Spain.Eur J Hosp Pharm. 2020 Nov;27(6):355-360. doi: 10.1136/ejhpharm-2018-001833. Epub 2019 May 7. Eur J Hosp Pharm. 2020. PMID: 33097619 Free PMC article.
-
A medicoeconomic review of early intervention with biologic agents in the treatment of inflammatory bowel diseases.Clinicoecon Outcomes Res. 2014 Oct 8;6:431-43. doi: 10.2147/CEOR.S39212. eCollection 2014. Clinicoecon Outcomes Res. 2014. PMID: 25336980 Free PMC article. Review.
References
-
- Friedman S, Blumberg RS. In: Harrison's online. 17. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, et al, editor. Chapter 276. New York: McGraw-Hill Health Professions Division; 2008. Inflammatory bowel disease. Accessed 2008 Feb 26.
-
- Tremaine WJ. Refractory IBD: medical management. Neth J Med. 1997;50(2):S12–S14. - PubMed
-
- Wong JLH. Clinic handbook: gastroenterology, [electronic resource] Chapter 11. Abingdon (UK): Taylor & Francis; 2002. Inflammatory bowel disease.
-
- Naber AH, de Jong DJ. Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials. Neth J Med. 2003;61(4):105–10. Available: PM:12852718. - PubMed
-
- Remicade®. e-CPS [database online] Ottawa: Canadian Pharmacists Association; 2008.
LinkOut - more resources
Full Text Sources