A systematic review of the reporting of Data Monitoring Committees' roles, interim analysis and early termination in pediatric clinical trials
- PMID: 20003383
- PMCID: PMC2801486
- DOI: 10.1186/1471-2431-9-77
A systematic review of the reporting of Data Monitoring Committees' roles, interim analysis and early termination in pediatric clinical trials
Abstract
Background: Decisions about interim analysis and early stopping of clinical trials, as based on recommendations of Data Monitoring Committees (DMCs), have far reaching consequences for the scientific validity and clinical impact of a trial. Our aim was to evaluate the frequency and quality of the reporting on DMC composition and roles, interim analysis and early termination in pediatric trials.
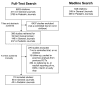
Methods: We conducted a systematic review of randomized controlled clinical trials published from 2005 to 2007 in a sample of four general and four pediatric journals. We used full-text databases to identify trials which reported on DMCs, interim analysis or early termination, and included children or adolescents. Information was extracted on general trial characteristics, risk of bias, and a set of parameters regarding DMC composition and roles, interim analysis and early termination.
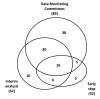
Results: 110 of the 648 pediatric trials in this sample (17%) reported on DMC or interim analysis or early stopping, and were included; 68 from general and 42 from pediatric journals. The presence of DMCs was reported in 89 of the 110 included trials (81%); 62 papers, including 46 of the 89 that reported on DMCs (52%), also presented information about interim analysis. No paper adequately reported all DMC parameters, and nine (15%) reported all interim analysis details. Of 32 trials which terminated early, 22 (69%) did not report predefined stopping guidelines and 15 (47%) did not provide information on statistical monitoring methods.
Conclusions: Reporting on DMC composition and roles, on interim analysis results and on early termination of pediatric trials is incomplete and heterogeneous. We propose a minimal set of reporting parameters that will allow the reader to assess the validity of trial results.
Figures
References
-
- Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, Elbourne DR, McLeer SK, Parmar MK, Pocock SJ. Issues in data monitoring and interim analysis of trials. Health Technol Assess. 2005;9:1–iv. - PubMed
-
- DAMOCLES Study Group. A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet. 2005;365:711–722. - PubMed
-
- European Medicines Agency (Committee for Medicinal Products for Human Use) Guideline on Data Monitoring Committees. 2005. http://www.emea.europa.eu/pdfs/human/ewp/587203en.pdf
-
- Food and Drug Administration. Establishment and Operation of Clinical Trial Data Monitoring Committees. 2006. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM127073.pdf
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

