An improved phage-display panning method to produce an HM-1 killer toxin anti-idiotypic antibody
- PMID: 20003392
- PMCID: PMC2801674
- DOI: 10.1186/1472-6750-9-99
An improved phage-display panning method to produce an HM-1 killer toxin anti-idiotypic antibody
Abstract
Background: Phage-display panning is an integral part of biomedical research. Regular panning methods are sometimes complicated by inefficient detachment of the captured phages from the antigen-coated solid supports, which prompted us to modify. Here, we produce an efficient antigen-specific single chain fragment variable (scFv) antibody by using a target-related molecule that favored selection of recombinant antibodies.
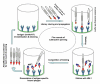
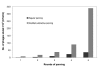
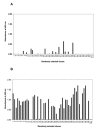
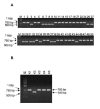
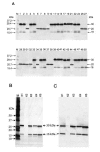
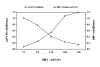
Results: To produce more selective and specific anti-idiotypic scFv-antibodies from a cDNA library, constructed from HM-1 killer toxin (HM-1)-neutralizing monoclonal antibodies (nmAb-KT), the method was modified by using an elution buffer supplemented with HM-1 that shares structural and functional similarities with the active site of the scFv antibody. Competitive binding of HM-1 to nmAb-KT allowed easy and quick dissociation of scFv-displayed phages from immobilized nmAb-KT to select specific anti-idiotypic scFv antibodies of HM-1. After modified panning, 80% clones (40/50) showed several times higher binding affinity to nmAb-KT than regular panning. The major populations (48%) of these clones (scFv K1) were genotypically same and had strong cytocidal activity against Saccharomyces and Candida species. The scFv K1 (K(d) value = 4.62 x 10(-8) M) had strong reactivity toward nmAb-KT, like HM-1 (K(d) value = 6.74 x 10(-9) M) as judged by SPR analysis.
Conclusion: The scFv antibodies generated after modified subtractive panning appear to have superior binding properties and cytocidal activity than regular panning. A simple modification of the elution condition in the phage-display panning protocol makes a large difference in determining success. Our method offers an attractive platform to discover potential therapeutic candidates.
Figures


Similar articles
-
Cloning antifungal single chain fragment variable antibodies by phage display and competitive panning elution.Anal Biochem. 2009 Dec 1;395(1):16-24. doi: 10.1016/j.ab.2009.08.003. Epub 2009 Aug 7. Anal Biochem. 2009. PMID: 19665444
-
An altered camelid-like single domain anti-idiotypic antibody fragment of HM-1 killer toxin: acts as an effective antifungal agent.Appl Microbiol Biotechnol. 2011 Apr;90(2):553-64. doi: 10.1007/s00253-011-3123-8. Epub 2011 Feb 9. Appl Microbiol Biotechnol. 2011. PMID: 21305279
-
Different buffer effects in selecting HM-1 killer toxin single-chain fragment variable anti-idiotypic antibodies.J Biochem. 2010 May;147(5):723-33. doi: 10.1093/jb/mvq006. Epub 2010 Jan 22. J Biochem. 2010. PMID: 20097900
-
[Development of anti-tumor blood vessel antibodies by phage display method].Yakugaku Zasshi. 2010 Apr;130(4):479-85. doi: 10.1248/yakushi.130.479. Yakugaku Zasshi. 2010. PMID: 20371989 Review. Japanese.
-
Antibody Selection via Phage Display in Microtiter Plates.Methods Mol Biol. 2023;2702:247-260. doi: 10.1007/978-1-0716-3381-6_12. Methods Mol Biol. 2023. PMID: 37679623 Review.
Cited by
-
Naïve Human Antibody Libraries for Infectious Diseases.Adv Exp Med Biol. 2017;1053:35-59. doi: 10.1007/978-3-319-72077-7_3. Adv Exp Med Biol. 2017. PMID: 29549634 Free PMC article. Review.
-
The isolation of novel phage display-derived human recombinant antibodies against CCR5, the major co-receptor of HIV.Viral Immunol. 2013 Aug;26(4):277-90. doi: 10.1089/vim.2012.0029. Viral Immunol. 2013. PMID: 23941674 Free PMC article.
-
The potential applications of T cell receptor (TCR)-like antibody in cervical cancer immunotherapy.Hum Vaccin Immunother. 2021 Sep 2;17(9):2981-2994. doi: 10.1080/21645515.2021.1913960. Epub 2021 May 14. Hum Vaccin Immunother. 2021. PMID: 33989511 Free PMC article. Review.
-
Bioprotective Role of Yeasts.Microorganisms. 2015 Oct 10;3(4):588-611. doi: 10.3390/microorganisms3040588. Microorganisms. 2015. PMID: 27682107 Free PMC article. Review.
-
Progress on Phage Display Technology: Tailoring Antibodies for Cancer Immunotherapy.Viruses. 2023 Sep 9;15(9):1903. doi: 10.3390/v15091903. Viruses. 2023. PMID: 37766309 Free PMC article. Review.
References
-
- Kordossis T, Avlami A, Velegraki A, Stefanou I, Georgakopoulos G, Papalambrou C, Legakis NJ. First report of Cryptococcus laurentii meningitis and a fatal case of Cryptococcus albidus cryptococcaemia in AIDS patients. Med Mycol. 1998;36:335–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

