Aqueous extracts from dietary supplements influence the production of inflammatory cytokines in immortalized and primary T lymphocytes
- PMID: 20003431
- PMCID: PMC2799390
- DOI: 10.1186/1472-6882-9-51
Aqueous extracts from dietary supplements influence the production of inflammatory cytokines in immortalized and primary T lymphocytes
Abstract
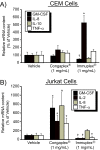
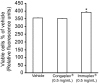
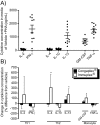
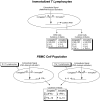
Background: Congaplex and Immuplex are dietary supplements that have been traditionally used to support immune system function. The purpose of these experiments was to determine whether Congaplex and Immuplex affect immune function using primary and immortalized T lymphocytes.
Methods: Immortalized CEM and Jurkat T lymphocytes and primary peripheral mononuclear blood cells (PBMCs) were treated with the aqueous extracts from Congaplex and Immuplex to determine the effects of these products on cytokine production in activated T lymphocytes.
Results: Congaplex enhanced phytohemagglutinin/phorbol 12-myristate 13-acetate (PHA/PMA) stimulation of both CEM and Jurkat cells as measured by the production of cytokines, while Immuplex suppressed PHA/PMA-induced production of cytokines, with the exception of interleukin (IL)-8 which was enhanced by Immuplex. In vitro treatment of PBMCs from 10 healthy donors with Congaplex or Immuplex decreased PHA-stimulated production of interferon (IFN)-gamma but increased the production of IL-13.
Conclusions: While the effects of Congaplex and Immuplex differed in these two models, these data demonstrate that the aqueous extracts from these two dietary supplements can affect the inflammatory response of T lymphocytes.
Figures




Similar articles
-
Gangliosides GD1b, GT1b, and GQ1b enhance IL-2 and IFN-gamma production and suppress IL-4 and IL-5 production in phytohemagglutinin-stimulated human T cells.J Immunol. 2001 Jan 1;166(1):72-80. doi: 10.4049/jimmunol.166.1.72. J Immunol. 2001. PMID: 11123278
-
Three-color flow cytometry detection of intracellular cytokines in peripheral blood mononuclear cells: comparative analysis of phorbol myristate acetate-ionomycin and phytohemagglutinin stimulation.Clin Diagn Lab Immunol. 2001 Mar;8(2):303-13. doi: 10.1128/CDLI.8.2.303-313.2001. Clin Diagn Lab Immunol. 2001. PMID: 11238213 Free PMC article.
-
Signal-dependent pleiotropic regulation of lymphocyte proliferation and cytokine production by 1,25-dihydroxyvitamin D3: potent modulation of the hormonal effects by phorbol esters.Immunology. 1992 Dec;77(4):520-6. Immunology. 1992. PMID: 1493924 Free PMC article.
-
Differences in the induction of induced human CD4(+) CD25(+) FoxP3(+) T-regulatory cells and CD3(+) CD8(+) CD28(-) T-suppressor cells subset phenotypes in vitro: comparison of phorbol 12-myristate 13-acetate/ionomycin and phytohemagglutinin stimulation.Transplant Proc. 2013 Jun;45(5):1822-31. doi: 10.1016/j.transproceed.2012.10.061. Transplant Proc. 2013. PMID: 23769052
-
Photoactivational cytokine-modulatory action of 8-methoxypsoralen plus ultraviolet A in lymphocytes, monocytes, and cutaneous T cell lymphoma cells.Ann N Y Acad Sci. 2001 Sep;941:185-93. doi: 10.1111/j.1749-6632.2001.tb03722.x. Ann N Y Acad Sci. 2001. PMID: 11594572 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

