An evolved xylose transporter from Zymomonas mobilis enhances sugar transport in Escherichia coli
- PMID: 20003468
- PMCID: PMC2801659
- DOI: 10.1186/1475-2859-8-66
An evolved xylose transporter from Zymomonas mobilis enhances sugar transport in Escherichia coli
Abstract
Background: Xylose is a second most abundant sugar component of lignocellulose besides glucose. Efficient fermentation of xylose is important for the economics of biomass-based biorefineries. However, sugar mixtures are sequentially consumed in xylose co-fermentation with glucose due to carbon catabolite repression (CCR) in microorganisms. As xylose transmembrance transport is one of the steps repressed by CCR, it is therefore of interest to develop a transporter that is less sensitive to the glucose inhibition or CCR.
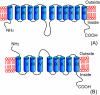
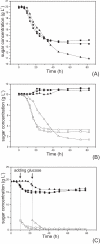
Results: The glucose facilitator protein Glf transporter from Zymomonas mobilis, also an efficient transporter for xylose, was chosen as the target transporter for engineering to eliminate glucose inhibition on xylose uptake. The evolution of Glf transporter was carried out with a mixture of glucose and xylose in E. coli. Error-prone PCR and random deletion were employed respectively in two rounds of evolution. Aided by a high-throughput screening assay using xylose analog p-nitrophenyl-beta-D-xylopyranoside (pNPX) in 96-well plates, a best mutant 2-RD5 was obtained that contains several mutations, and a deletion of 134 residues (about 28% of total residues), or three fewer transmembrane sections (TMSs). It showed a 10.8-fold improvement in terms of pNPX transport activity in the presence of glucose. The fermentation performance results showed that this mutant improved xylose consumption by 42% with M9 minimal medium containing 20 g L-1 xylose only, while with the mixture sugar of xylose and glucose, 28% more glucose was consumed, but no obvious co-utilization of xylose was observed. Further glucose fed-batch experiments suggested that the intracellular metabolism of xylose was repressed by glucose.
Conclusions: Through random mutagenesis and partial deletion coupled with high-throughput screening, a mutant of the Glf transporter (2-RD5) was obtained that relieved the inhibition of xylose transport by glucose. The fermentation tests revealed that 2-RD5 was advantageous in xylose and glucose uptakes, while no obvious advantage was seen for xylose co-consumption when co-fermented with glucose. Further efforts could focus on reducing CCR-mediated repression of intracellular metabolism of xylose. Glf should also serve as a useful model to further exploit the molecular mechanism of xylose transport and the CCR-mediated inhibition.
Figures


References
-
- Zverlov VV, Berezina O, Velikodvorskaya GA, Schwarz WH. Bacterial acetone and butanol production by industrial fermentation in the Soviet Union: use of hydrolyzed agricultural waste for biorefinery. Applied Microbiology and Biotechnology. 2006;71:587–597. doi: 10.1007/s00253-006-0445-z. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

