Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis state of the art
- PMID: 20003476
- PMCID: PMC2805599
- DOI: 10.1186/1755-1536-2-7
Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis state of the art
Abstract
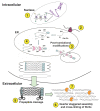
Fibrosis represents a major global disease burden, yet a potent antifibrotic compound is still not in sight. Part of the explanation for this situation is the difficulties that both academic laboratories and research and development departments in the pharmaceutical industry have been facing in re-enacting the fibrotic process in vitro for screening procedures prior to animal testing. Effective in vitro characterization of antifibrotic compounds has been hampered by cell culture settings that are lacking crucial cofactors or are not holistic representations of the biosynthetic and depositional pathway leading to the formation of an insoluble pericellular collagen matrix. In order to appreciate the task which in vitro screening of antifibrotics is up against, we will first review the fibrotic process by categorizing it into events that are upstream of collagen biosynthesis and the actual biosynthetic and depositional cascade of collagen I. We point out oversights such as the omission of vitamin C, a vital cofactor for the production of stable procollagen molecules, as well as the little known in vitro tardy procollagen processing by collagen C-proteinase/BMP-1, another reason for minimal collagen deposition in cell culture. We review current methods of cell culture and collagen quantitation vis-à-vis the high content options and requirements for normalization against cell number for meaningful data retrieval. Only when collagen has formed a fibrillar matrix that becomes cross-linked, invested with ligands, and can be remodelled and resorbed, the complete picture of fibrogenesis can be reflected in vitro. We show here how this can be achieved. A well thought-out in vitro fibrogenesis system represents the missing link between brute force chemical library screens and rational animal experimentation, thus providing both cost-effectiveness and streamlined procedures towards the development of better antifibrotic drugs.
Figures
References
-
- WHO. The Global Burden of Disease 2004 Update. Geneva: World Health Organization; 2004.
-
- Schmermund A, Schwartz RS, Adamzik M, Sangiorgi G, Pfeifer EA, Rumberger JA, Burke AP, Farb A, Virmani R. Coronary atherosclerosis in unheralded sudden coronary death under age 50: histo-pathologic comparison with 'healthy' subjects dying out of hospital. Atherosclerosis. 2001;155:499–508. doi: 10.1016/S0021-9150(00)00598-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

