A transcriptomic computational analysis of mastic oil-treated Lewis lung carcinomas reveals molecular mechanisms targeting tumor cell growth and survival
- PMID: 20003503
- PMCID: PMC2801511
- DOI: 10.1186/1755-8794-2-68
A transcriptomic computational analysis of mastic oil-treated Lewis lung carcinomas reveals molecular mechanisms targeting tumor cell growth and survival
Abstract
Background: Mastic oil from Pistacia lentiscus variation chia, a blend of bioactive terpenes with recognized medicinal properties, has been recently shown to exert anti-tumor growth activity through inhibition of cancer cell proliferation, survival, angiogenesis and inflammatory response. However, no studies have addressed its mechanisms of action at genome-wide gene expression level.
Methods: To investigate molecular mechanisms triggered by mastic oil, Lewis Lung Carcinoma cells were treated with mastic oil or DMSO and RNA was collected at five distinct time points (3-48 h). Microarray expression profiling was performed using Illumina mouse-6 v1 beadchips, followed by computational analysis. For a number of selected genes, RT-PCR validation was performed in LLC cells as well as in three human cancer cell lines of different origin (A549, HCT116, K562). PTEN specific inhibition by a bisperovanadium compound was applied to validate its contribution to mastic oil-mediated anti-tumor growth effects.
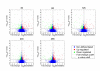
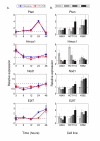
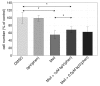
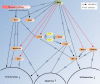
Results: In this work we demonstrated that exposure of Lewis lung carcinomas to mastic oil caused a time-dependent alteration in the expression of 925 genes. GO analysis associated expression profiles with several biological processes and functions. Among them, modifications on cell cycle/proliferation, survival and NF-kappaB cascade in conjunction with concomitant regulation of genes encoding for PTEN, E2F7, HMOX1 (up-regulation) and NOD1 (down-regulation) indicated some important mechanistic links underlying the anti-proliferative, pro-apoptotic and anti-inflammatory effects of mastic oil. The expression profiles of Hmox1, Pten and E2f7 genes were similarly altered by mastic oil in the majority of test cancer cell lines. Inhibition of PTEN partially reversed mastic oil effects on tumor cell growth, indicating a multi-target mechanism of action. Finally, k-means clustering, organized the significant gene list in eight clusters demonstrating a similar expression profile. Promoter analysis in a representative cluster revealed shared putative cis-elements suggesting a common regulatory transcription mechanism.
Conclusions: Present results provide novel evidence on the molecular basis of tumor growth inhibition mediated by mastic oil and set a rational basis for application of genomics and bioinformatic methodologies in the screening of natural compounds with potential cancer chemopreventive activities.
Figures


References
-
- American Institute for Cancer Research, World Cancer Research Fund. Food, nutrition, physical activity and the prevention of cancer: a global perspective: a project of World Cancer Research Fund International. Washington, D.C.: American Institute for Cancer Research; 2007.
-
- Gopalakrishnan A, Tony Kong AN. Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells. Food Chem Toxicol. 2008;46(4):1257–1270. doi: 10.1016/j.fct.2007.09.082. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

