Unrestricted somatic stem cells from human umbilical cord blood grow in serum-free medium as spheres
- PMID: 20003538
- PMCID: PMC2805630
- DOI: 10.1186/1472-6750-9-101
Unrestricted somatic stem cells from human umbilical cord blood grow in serum-free medium as spheres
Abstract
Background: Human umbilical cord blood-derived unrestricted somatic stem cells (USSCs), which are capable of multilineage differentiation, are currently under investigation for a number of therapeutic applications. A major obstacle to their clinical use is the fact that in vitro expansion is still dependent upon fetal calf serum, which could be a source of pathogens. In this study, we investigate the capacity of three different stem cell culture media to support USSCs in serum-free conditions; HEScGRO, PSM and USSC growth medium ACF. Our findings demonstrate that USSCs do not grow in HEScGRO or PSM, but we were able to isolate, proliferate and maintain multipotency of three USSC lines in USSC growth medium ACF.
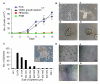
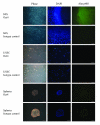
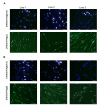
Results: For the first one to three passages, cells grown in USSC growth medium ACF proliferate and maintain their morphology, but with continued passaging the cells form spherical cell aggregates. Upon dissociation of spheres, cells continue to grow in suspension and form new spheres. Dissociated cells can also revert to monolayer growth when cultured on extracellular matrix support (fibronectin or gelatin), or in medium containing fetal calf serum. Analysis of markers associated with pluripotency (Oct4 and Sox2) and differentiation (FoxA2, Brachyury, Goosecoid, Nestin, Pax6, Gata6 and Cytokeratin 8) confirms that cells in the spheres maintain their gene expression profile. The cells in the spheres also retain the ability to differentiate in vitro to form cells representative of the three germline layers after five passages.
Conclusions: These data suggest that USSC growth medium ACF maintains USSCs in an undifferentiated state and supports growth in suspension. This is the first demonstration that USSCs can grow in a serum- and animal component-free medium and that USSCs can form spheres.
Figures

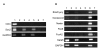




References
-
- Kim BO, Tian H, Prasongsukarn K, Wu J, Angoulvant D, Wnendt S, Muhs A, Spitkovsky D, Li RK. Cell transplantation improves ventricular function after a myocardial infarction: a preclinical study of human unrestricted somatic stem cells in a porcine model. Circulation. 2005;112(9 Suppl):I96–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

