Advances and challenges in malaria vaccine development
- PMID: 20003658
- PMCID: PMC2943423
- DOI: 10.1017/S1462399409001318
Advances and challenges in malaria vaccine development
Abstract
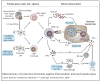
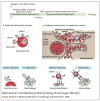
Malaria remains one of the most devastating infectious diseases that threaten humankind. Human malaria is caused by five different species of Plasmodium parasites, each transmitted by the bite of female Anopheles mosquitoes. Plasmodia are eukaryotic protozoans with more than 5000 genes and a complex life cycle that takes place in the mosquito vector and the human host. The life cycle can be divided into pre-erythrocytic stages, erythrocytic stages and mosquito stages. Malaria vaccine research and development faces formidable obstacles because many vaccine candidates will probably only be effective in a specific species at a specific stage. In addition, Plasmodium actively subverts and escapes immune responses, possibly foiling vaccine-induced immunity. Although early successful vaccinations with irradiated, live-attenuated malaria parasites suggested that a vaccine is possible, until recently, most efforts have focused on subunit vaccine approaches. Blood-stage vaccines remain a primary research focus, but real progress is evident in the development of a partially efficacious recombinant pre-erythrocytic subunit vaccine and a live-attenuated sporozoite vaccine. It is unlikely that partially effective vaccines will eliminate malaria; however, they might prove useful in combination with existing control strategies. Elimination of malaria will probably ultimately depend on the development of highly effective vaccines.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

