Translational modulation of proteins expressed from bicistronic vectors
- PMID: 20003889
- PMCID: PMC2864087
Translational modulation of proteins expressed from bicistronic vectors
Abstract
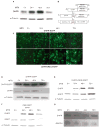
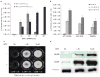
Bicistronic vectors are useful tools for exogenous expression of two gene products from a single promoter element; however, reduced expression of protein from the second cistron compared with the first cistron is a common limitation to this approach. To overcome this limitation, we explored use of dihydrofolate reductase (DHFR) complementary DNA encoded in bicistronic vectors to induce a second protein of interest by methotrexate (MTX) treatment. Previous studies have demonstrated that levels of DHFR protein and DHFR fusion protein can be induced translationally following MTX treatment of cells. We demonstrated that in response to MTX treatment, DHFR partner protein in a bicistronic construct is induced for longer periods of time when compared with endogenous DHFR and DHFR fusion protein, in vitro and in vivo. Using rapamycin pretreatment followed by MTX treatment, we also devised a strategy to modulate levels of two proteins expressed from a bicistronic construct in a cap-independent manner. To our knowledge, this is the first report demonstrating that levels of proteins in DHFR-based bicistronic constructs can be induced and modulated using MTX and rapamycin treatment.
Figures
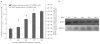

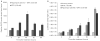


Similar articles
-
Retroviral transduction of a mutant dihydrofolate reductase-thymidylate synthase fusion gene into murine marrow cells confers resistance to both methotrexate and 5-fluorouracil.Hum Gene Ther. 2003 Mar 20;14(5):435-46. doi: 10.1089/104303403321467207. Hum Gene Ther. 2003. PMID: 12691609
-
Comparison of methotrexate resistance conferred by a mutated dihydrofolate reductase (DHFR) cDNA in two different retroviral vectors.Cancer Gene Ther. 2000 Jun;7(6):910-9. doi: 10.1038/sj.cgt.7700199. Cancer Gene Ther. 2000. PMID: 10880023
-
Coexpression of cytidine deaminase and mutant dihydrofolate reductase by a bicistronic retroviral vector confers resistance to cytosine arabinoside and methotrexate.Hum Gene Ther. 1998 Nov 20;9(17):2537-44. doi: 10.1089/hum.1998.9.17-2537. Hum Gene Ther. 1998. PMID: 9853520
-
Molecular mechanisms of resistance to antifolates, a review.Acta Biochim Pol. 1995;42(4):457-64. Acta Biochim Pol. 1995. PMID: 8852336 Review.
-
Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):164-73. doi: 10.1016/s0925-4439(02)00079-0. Biochim Biophys Acta. 2002. PMID: 12084458 Review.
Cited by
-
Modulation of immunogenicity and immunoprotection of mucosal vaccine against coxsackievirus B3 by optimizing the coadministration mode of lymphotactin adjuvant.DNA Cell Biol. 2012 Apr;31(4):479-88. doi: 10.1089/dna.2011.1367. Epub 2011 Oct 11. DNA Cell Biol. 2012. PMID: 21988406 Free PMC article.
-
Treatment of a solid tumor using engineered drug-resistant immunocompetent cells and cytotoxic chemotherapy.Hum Gene Ther. 2012 Jul;23(7):711-21. doi: 10.1089/hum.2011.172. Epub 2012 Apr 18. Hum Gene Ther. 2012. PMID: 22397715 Free PMC article.
References
-
- Pyronnet S, Pradayrol L, Sonenberg N. A cell cycle-dependent internal ribosome entry site. Mol Cell. 2000;5:607–16. - PubMed
-
- Bochkov YA, Palmenberg AC. Translational efficiency of EMCV IRES in bicistronic vectors is dependent upon IRES sequence and gene location. Biotechniques. 2006;41:283–4. 286, 288. - PubMed
-
- Mitchell SA, Spriggs KA, Coldwell MJ, et al. The Apaf-1 internal ribosome entry segment attains the correct structural conformation for function via interactions with PTB and unr. Mol Cell. 2003;11:757–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
