Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior
- PMID: 20004171
- PMCID: PMC2819560
- DOI: 10.1016/j.abb.2009.12.005
Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior
Abstract
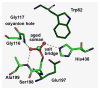
Butyrylcholinesterase is a promiscuous enzyme that displays complex kinetic behavior. It is toxicologically important because it detoxifies organophosphorus poisons (OP) by making a covalent bond with the OP. The OP and the butyrylcholinesterase are both inactivated in the process. Inactivation of butyrylcholinesterase has no adverse effects. However, inactivation of acetylcholinesterase in nerve synapses can be lethal. OP-inhibited butyrylcholinesterase and acetylcholinesterase can be reactivated with oximes provided the OP has not aged. Strategies for preventing the toxicity of OP include (a) treatment with an OP scavenger, (b) reaction of non-aged enzyme with oximes, (c) reactivation of aged enzyme, (d) slowing down aging with peripheral site ligands, and (e) design of mutants that rapidly hydrolyze OP. Option (a) has progressed through phase I clinical trials with human butyrylcholinesterase. Option (b) is in routine clinical use. The others are at the basic research level. Butyrylcholinesterase displays complex kinetic behavior including activation by positively charged esters, ability to hydrolyze amides, and a lag time (hysteresis) preceding hydrolysis of benzoylcholine and N-methylindoxyl acetate. Mass spectrometry has identified new OP binding motifs on tyrosine and lysine in proteins that have no active site serine. It is proposed, but not yet proven, that low dose exposure involves OP modification of proteins that have no active site serine.
2009 Elsevier Inc. All rights reserved.
Figures
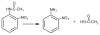

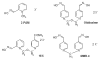
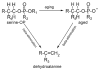


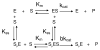
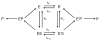
References
-
- Saxena A, Sun W, Luo C, Myers TM, Koplovitz I, Lenz DE, Doctor BP. J Mol Neurosci. 2006;30:145–148. - PubMed
-
- Manoharan I, Boopathy R, Darvesh S, Lockridge O. Clin Chim Acta. 2007;378:128–135. - PubMed
-
- Darvesh S, Hopkins DA. J Comp Neurol. 2003;463:25–43. - PubMed
-
- Wetherell JR, French MC. Biochem Pharmacol. 1986;35:939–945. - PubMed
-
- Duysen EG, Li B, Xie W, Schopfer LM, Anderson RS, Broomfield CA, Lockridge O. J Pharmacol Exp Ther. 2001;299:528–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

