Light activation of rhodopsin: insights from molecular dynamics simulations guided by solid-state NMR distance restraints
- PMID: 20004206
- PMCID: PMC2822010
- DOI: 10.1016/j.jmb.2009.12.003
Light activation of rhodopsin: insights from molecular dynamics simulations guided by solid-state NMR distance restraints
Abstract
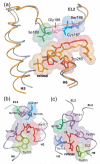
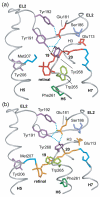
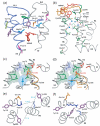
Structural restraints provided by solid-state NMR measurements of the metarhodopsin II intermediate are combined with molecular dynamics simulations to help visualize structural changes in the light activation of rhodopsin. Since the timescale for the formation of the metarhodopsin II intermediate (>1 ms) is beyond that readily accessible by molecular dynamics, we use NMR distance restraints derived from 13C dipolar recoupling measurements to guide the simulations. The simulations yield a working model for how photoisomerization of the 11-cis retinylidene chromophore bound within the interior of rhodopsin is coupled to transmembrane helix motion and receptor activation. The mechanism of activation that emerges is that multiple switches on the extracellular (or intradiscal) side of rhodopsin trigger structural changes that converge to disrupt the ionic lock between helices H3 and H6 on the intracellular side of the receptor.
Copyright (c) 2009. Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Location of Trp265 in metarhodopsin II: implications for the activation mechanism of the visual receptor rhodopsin.J Mol Biol. 2006 Mar 17;357(1):163-72. doi: 10.1016/j.jmb.2005.12.046. Epub 2006 Jan 3. J Mol Biol. 2006. PMID: 16414074
-
Structural transitions of transmembrane helix 6 in the formation of metarhodopsin I.J Phys Chem B. 2012 Sep 6;116(35):10477-89. doi: 10.1021/jp3019183. Epub 2012 May 17. J Phys Chem B. 2012. PMID: 22564141 Free PMC article.
-
Helix movement is coupled to displacement of the second extracellular loop in rhodopsin activation.Nat Struct Mol Biol. 2009 Feb;16(2):168-75. doi: 10.1038/nsmb.1549. Epub 2009 Feb 1. Nat Struct Mol Biol. 2009. PMID: 19182802 Free PMC article.
-
Retinal dynamics during light activation of rhodopsin revealed by solid-state NMR spectroscopy.Biochim Biophys Acta. 2010 Feb;1798(2):177-93. doi: 10.1016/j.bbamem.2009.08.013. Epub 2009 Aug 28. Biochim Biophys Acta. 2010. PMID: 19716801 Free PMC article. Review.
-
Retinal conformation and dynamics in activation of rhodopsin illuminated by solid-state H NMR spectroscopy.Photochem Photobiol. 2009 Mar-Apr;85(2):442-53. doi: 10.1111/j.1751-1097.2008.00510.x. Photochem Photobiol. 2009. PMID: 19267870 Free PMC article. Review.
Cited by
-
Sequential rearrangement of interhelical networks upon rhodopsin activation in membranes: the Meta II(a) conformational substate.J Am Chem Soc. 2010 Apr 7;132(13):4815-21. doi: 10.1021/ja910317a. J Am Chem Soc. 2010. PMID: 20230054 Free PMC article.
-
Retinal Conformation Changes Rhodopsin's Dynamic Ensemble.Biophys J. 2015 Aug 4;109(3):608-17. doi: 10.1016/j.bpj.2015.06.046. Biophys J. 2015. PMID: 26244742 Free PMC article.
-
Modulation of constitutive activity and signaling bias of the ghrelin receptor by conformational constraint in the second extracellular loop.J Biol Chem. 2012 Sep 28;287(40):33488-502. doi: 10.1074/jbc.M112.383240. Epub 2012 Jul 30. J Biol Chem. 2012. PMID: 22846991 Free PMC article.
-
Identification of essential cannabinoid-binding domains: structural insights into early dynamic events in receptor activation.J Biol Chem. 2011 Sep 23;286(38):33422-35. doi: 10.1074/jbc.M111.261651. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795705 Free PMC article.
-
Retinal ligand mobility explains internal hydration and reconciles active rhodopsin structures.Biochemistry. 2014 Jan 21;53(2):376-85. doi: 10.1021/bi4013947. Epub 2014 Jan 8. Biochemistry. 2014. PMID: 24328554 Free PMC article.
References
-
- Menon ST, Han M, Sakmar TP. Rhodopsin: Structural basis of molecular physiology. Physiol. Rev. 2001;81:1659–1688. - PubMed
-
- Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7TM receptor activation - A global toggle switch model. Annu. Rev. Pharmacol. Toxicol. 2006;46:481–519. - PubMed
-
- Kobilka B, Schertler GFX. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol. Sci. 2008;29:79–83. - PubMed
-
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. - PubMed
-
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J. Mol. Biol. 2004;342:571–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

