The effects of acute and chronic ethanol exposure on presynaptic and postsynaptic gamma-aminobutyric acid (GABA) neurotransmission in cultured cortical and hippocampal neurons
- PMID: 20004338
- PMCID: PMC2796564
- DOI: 10.1016/j.alcohol.2009.10.006
The effects of acute and chronic ethanol exposure on presynaptic and postsynaptic gamma-aminobutyric acid (GABA) neurotransmission in cultured cortical and hippocampal neurons
Abstract
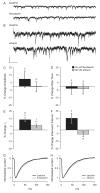
Decades after ethanol was first described as a gamma-aminobutyric acid (GABA) mimetic, the precise mechanisms that produce the acute effects of ethanol and the physiological adaptations that underlie ethanol tolerance and dependence remain unclear. Although a substantial body of evidence suggests that ethanol acts on GABAergic neurotransmission to enhance inhibition in the central nervous system, the precise mechanisms underlying the physiological effects of both acute and chronic ethanol exposure are still under investigation. We have used in vitro ethanol exposure followed by recording of miniature inhibitory postsynaptic currents (mIPSCs) to determine whether acute or chronic ethanol exposure directly alters synaptic GABA(A) receptor (GABA(A)R) function or GABA release in cultured cortical and hippocampal neurons. Acute ethanol exposure slightly increased the duration of mIPSCs in hippocampal neurons but did not alter mIPSC kinetics in cortical neurons. Acute ethanol exposure did not change mIPSC frequency in either hippocampal or cortical neurons. One day of chronic ethanol exposure produced a transient decrease in mIPSC duration in cortical neurons but did not alter mIPSC kinetics in hippocampal neurons. Chronic ethanol exposure did not change mIPSC frequency in either hippocampal or cortical neurons. Chronic ethanol exposure also did not produce substantial cross-tolerance to a benzodiazepine in either hippocampal or cortical neurons. The results suggest that ethanol exposure in vitro has limited effects on synaptic GABA(A)R function and action potential-independent GABA release in cultured neurons and that ethanol exposure in cultured cortical and hippocampal neurons may not reproduce all the effects that occur in vivo and in acute brain slices.
Figures





References
-
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl− current in mouse hippocampal and cortical neurons. Eur. J. Pharmacol. 1990;187:127–130. - PubMed
-
- Bouron A. Modulation of spontaneous quantal release of neurotransmitters in the hippocampus. Prog. Neurobiol. 2001;63:613–635. - PubMed
-
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol. Pharmacol. 2003;63:53–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

