The phosphatase Shp2 is required for signaling by the Kaposi's sarcoma-associated herpesvirus viral GPCR in primary endothelial cells
- PMID: 20004456
- PMCID: PMC2822116
- DOI: 10.1016/j.virol.2009.11.030
The phosphatase Shp2 is required for signaling by the Kaposi's sarcoma-associated herpesvirus viral GPCR in primary endothelial cells
Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of Kaposi's sarcoma (KS), an AIDS-related endothelial cell malignancy that is the most common cancer in central and southern Africa. The KSHV viral G protein-coupled receptor (vGPCR) is a viral oncogene that conveys a survival advantage to endothelial cells and causes KS-like tumors in mouse models. In this study we investigate the role of Shp2, a protein tyrosine phosphatase in vGPCR signaling. Shp2 is vital to many cytokine-induced signaling pathways and is dysregulated in various infections and malignancies. It has also recently been implicated in angiogenesis. We find that vGPCR activity results in phosphorylation of regulatory tyrosines in Shp2 and that in turn, Shp2 is required for vGPCR-mediated activation of MEK, NFkappaB, and AP-1. Furthermore, both genetic and chemical inhibition of Shp2 abrogate vGPCR-induced enhancement of endothelial cell migration. This establishes Shp2 as an important point of convergence of KSHV vGPCR signaling and a potential molecular target in the design of an anti-KSHV therapeutic regimen.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
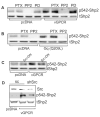


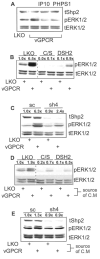
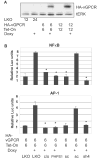
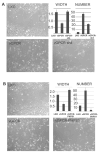
References
-
- Adachi M, Fischer EH, Ihle J, Imai K, Jirik F, Neel B, Pawson T, Shen S, Thomas M, Ullrich A, Zhao Z. Mammalian SH2-containing protein tyrosine phosphatases. Cell. 1996;85(1):15. - PubMed
-
- Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117(6):699–711. - PubMed
-
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385(6614):347–50. - PubMed
-
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391(6662):86–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

