Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial
- PMID: 20004464
- PMCID: PMC2805723
- DOI: 10.1016/S0140-6736(09)62067-5
Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial
Abstract
Background: HIV antiretroviral therapy (ART) is often managed without routine laboratory monitoring in Africa; however, the effect of this approach is unknown. This trial investigated whether routine toxicity and efficacy monitoring of HIV-infected patients receiving ART had an important long-term effect on clinical outcomes in Africa.
Methods: In this open, non-inferiority trial in three centres in Uganda and one in Zimbabwe, 3321 symptomatic, ART-naive, HIV-infected adults with CD4 counts less than 200 cells per microL starting ART were randomly assigned to laboratory and clinical monitoring (LCM; n=1659) or clinically driven monitoring (CDM; n=1662) by a computer-generated list. Haematology, biochemistry, and CD4-cell counts were done every 12 weeks. In the LCM group, results were available to clinicians; in the CDM group, results (apart from CD4-cell count) could be requested if clinically indicated and grade 4 toxicities were available. Participants switched to second-line ART after new or recurrent WHO stage 4 events in both groups, or CD4 count less than 100 cells per microL (LCM only). Co-primary endpoints were new WHO stage 4 HIV events or death, and serious adverse events. Non-inferiority was defined as the upper 95% confidence limit for the hazard ratio (HR) for new WHO stage 4 events or death being no greater than 1.18. Analyses were by intention to treat. This study is registered, number ISRCTN13968779.
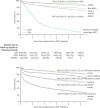
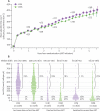
Findings: Two participants assigned to CDM and three to LCM were excluded from analyses. 5-year survival was 87% (95% CI 85-88) in the CDM group and 90% (88-91) in the LCM group, and 122 (7%) and 112 (7%) participants, respectively, were lost to follow-up over median 4.9 years' follow-up. 459 (28%) participants receiving CDM versus 356 (21%) LCM had a new WHO stage 4 event or died (6.94 [95% CI 6.33-7.60] vs 5.24 [4.72-5.81] per 100 person-years; absolute difference 1.70 per 100 person-years [0.87-2.54]; HR 1.31 [1.14-1.51]; p=0.0001). Differences in disease progression occurred from the third year on ART, whereas higher rates of switch to second-line treatment occurred in LCM from the second year. 283 (17%) participants receiving CDM versus 260 (16%) LCM had a new serious adverse event (HR 1.12 [0.94-1.32]; p=0.19), with anaemia the most common (76 vs 61 cases).
Interpretation: ART can be delivered safely without routine laboratory monitoring for toxic effects, but differences in disease progression suggest a role for monitoring of CD4-cell count from the second year of ART to guide the switch to second-line treatment.
Funding: UK Medical Research Council, the UK Department for International Development, the Rockefeller Foundation, GlaxoSmithKline, Gilead Sciences, Boehringer-Ingelheim, and Abbott Laboratories.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
DART points the way for HIV treatment programmes.Lancet. 2010 Jan 9;375(9709):96-8. doi: 10.1016/S0140-6736(09)62103-6. Epub 2009 Dec 8. Lancet. 2010. PMID: 20004465 No abstract available.
-
Paper of the year 2009: results.Lancet. 2010 Feb 20;375(9715):622-3. doi: 10.1016/S0140-6736(10)60246-2. Lancet. 2010. PMID: 20171386 No abstract available.
-
DART and laboratory monitoring of HIV treatment.Lancet. 2010 Mar 20;375(9719):979. doi: 10.1016/S0140-6736(10)60429-1. Lancet. 2010. PMID: 20304233 No abstract available.
-
DART and laboratory monitoring of HIV treatment.Lancet. 2010 Mar 20;375(9719):979; author reply 979-80. doi: 10.1016/S0140-6736(10)60430-8. Lancet. 2010. PMID: 20304235 No abstract available.
References
-
- UNAIDS The global economic crisis and HIV prevention and treatment programmes: vulnerabilities and impact. June, 2009. http://data.unaids.org/pub/Report/2009/jc1704_econcrisis_hivresponse_en.pdf (accessed Dec 2, 2009).
-
- World Bank Averting a human crisis during the global downturn: policy options from the World Bank's Human Development Network. 2009. http://siteresources.worldbank.org/NEWS/Resources/AvertingTheHumanCrisis... (accessed Dec 2, 2009).
-
- Gilks CF, Crowley S, Ekpini R. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. - PubMed
-
- Coutinho A, Mermin J, Ekwaru J, et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among HIV-infected adults in Uganda: a randomized trial. 15th Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA; Feb 3–6, 2008. Abstract 125.
-
- WHO . Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. Recommendations for a public health approach. World Health Organization; Geneva: 2006. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

