A multi-center, randomized, controlled trial of parenteral nutrition titrated to resting energy expenditure in children undergoing hematopoietic stem cell transplantation ("PNTREE"): rationale and design
- PMID: 20004739
- PMCID: PMC3635085
- DOI: 10.1016/j.cct.2009.12.002
A multi-center, randomized, controlled trial of parenteral nutrition titrated to resting energy expenditure in children undergoing hematopoietic stem cell transplantation ("PNTREE"): rationale and design
Abstract
Background: Children undergoing hematopoietic stem cell transplantation (HSCT) frequently require prolonged courses of parenteral nutrition (PN) as a consequence of gastrointestinal dysfunction related to preparative chemotherapy and radiation. PN has been associated with shorter engraftment time and decreased mortality during HSCT, however, it is also linked with complications, including infections, liver disease, and metabolic disturbances. Some of these complications may be a result of providing PN in excess of nutrient requirements. We previously described significant reductions in resting energy expenditure (REE), as measured by indirect calorimetry, over the course of HSCT. We also documented a decline in mid-arm muscle area, suggesting depletion of muscle mass, while triceps skinfold, a marker of fat stores, was unchanged. These results suggested the need for further study of energy expenditure, body composition and nutritional intake in this group of high risk patients.
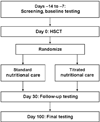
Design and hypothesis: We hypothesize that changes in body composition affect REE during HSCT, and that standard nutritional support may lead to overfeeding. We are performing a randomized controlled trial of parenteral nutrition among children undergoing allogeneic HSCT. Subjects are randomized to receive PN designed to provide 100% of measured REE, or standard PN, i.e., 140% of estimated energy expenditure. The primary outcome variable is change in percent body fat. Secondary outcomes include glycemic control and frequency of infections, changes in REE and body composition.
Conclusion: This study will provide unique and comprehensive nutritional data and its results will guide nutritional therapy for children undergoing HSCT and possibly other catabolic patients.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Attenuation of resting energy expenditure following hematopoietic SCT in children.Bone Marrow Transplant. 2012 Oct;47(10):1301-6. doi: 10.1038/bmt.2012.19. Epub 2012 Feb 20. Bone Marrow Transplant. 2012. PMID: 22343669 Free PMC article. Clinical Trial.
-
Effect of titrated parenteral nutrition on body composition after allogeneic hematopoietic stem cell transplantation in children: a double-blind, randomized, multicenter trial.Am J Clin Nutr. 2012 Feb;95(2):342-51. doi: 10.3945/ajcn.111.026005. Epub 2011 Dec 28. Am J Clin Nutr. 2012. PMID: 22205317 Free PMC article. Clinical Trial.
-
Changes in resting energy expenditure among children undergoing allogeneic stem cell transplantation.Am J Clin Nutr. 2003 Jul;78(1):104-9. doi: 10.1093/ajcn/78.1.104. Am J Clin Nutr. 2003. PMID: 12816778 Clinical Trial.
-
Nutritional and Post-Transplantation Outcomes of Enteral versus Parenteral Nutrition in Pediatric Hematopoietic Stem Cell Transplantation: A Systematic Review of Randomized and Nonrandomized Studies.Biol Blood Marrow Transplant. 2019 Aug;25(8):e252-e259. doi: 10.1016/j.bbmt.2019.02.023. Epub 2019 Mar 1. Biol Blood Marrow Transplant. 2019. PMID: 30826462
-
The effects of chemotherapy on energy metabolic aspects in cancer patients: A systematic review.Clin Nutr. 2020 Jun;39(6):1863-1877. doi: 10.1016/j.clnu.2019.07.028. Epub 2019 Aug 7. Clin Nutr. 2020. PMID: 31420208
Cited by
-
Protective Effects of 6-Gingerol on Cardiotoxicity Induced by Arsenic Trioxide Through AMPK/SIRT1/PGC-1α Signaling Pathway.Front Pharmacol. 2022 Apr 28;13:868393. doi: 10.3389/fphar.2022.868393. eCollection 2022. Front Pharmacol. 2022. PMID: 35571130 Free PMC article.
-
Bone loss and vitamin D deficiency in children undergoing hematopoietic cell transplantation.Pediatr Blood Cancer. 2015 Apr;62(4):687-92. doi: 10.1002/pbc.25370. Epub 2015 Jan 28. Pediatr Blood Cancer. 2015. PMID: 25630874 Free PMC article. Clinical Trial.
-
NRS2002 assesses nutritional status of leukemia patients undergoing hematopoietic stem cell transplantation.Chin J Cancer Res. 2012 Dec;24(4):299-303. doi: 10.3978/j.issn.1000-9604.2012.09.01. Chin J Cancer Res. 2012. PMID: 23359777 Free PMC article.
-
Attenuation of resting energy expenditure following hematopoietic SCT in children.Bone Marrow Transplant. 2012 Oct;47(10):1301-6. doi: 10.1038/bmt.2012.19. Epub 2012 Feb 20. Bone Marrow Transplant. 2012. PMID: 22343669 Free PMC article. Clinical Trial.
-
Effect of titrated parenteral nutrition on body composition after allogeneic hematopoietic stem cell transplantation in children: a double-blind, randomized, multicenter trial.Am J Clin Nutr. 2012 Feb;95(2):342-51. doi: 10.3945/ajcn.111.026005. Epub 2011 Dec 28. Am J Clin Nutr. 2012. PMID: 22205317 Free PMC article. Clinical Trial.
References
-
- Weisdorf S, Hofland C, Sharp H, et al. Total parenteral nutrition in bone marrow transplantation: a clinical evaluation. J Pediatr Gastroenterol Nutr. 1984;3:95–100. - PubMed
-
- Weisdorf S, Lysne J, Wind D, et al. Positive effect of prophylactic total parenteral nutrition on long-term outcome of bone marrow transplantation. Transplantation. 1987;43(6):833–838. - PubMed
-
- Kerner JA, Jr, Hurwitz M. Parenteral Nutrition. In: Duggan C, Watkins J, Walker WA, editors. Nutrition in pediatrics: basic science and clinical applications. 4th ed. Ontario, BC Decker: Hamilton; 2008. pp. 777–793.
-
- Chwals WJ. Overfeeding the critically ill child: fact or fantasy? New Horiz. 1994;2(2):147–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

