Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses
- PMID: 20004775
- PMCID: PMC2995268
- DOI: 10.1016/j.jaci.2009.11.016
Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses
Abstract
The discovery of the genetic causes of a rare group of immune-mediated inflammatory conditions that mimic infections and allergic conditions in their clinical presentation and the molecular understanding of the function of the mutated molecules in these diseases has led to a revolution in our understanding of the pathogenesis of systemic and local inflammation. The proteins mutated in a number of these so-called autoinflammatory diseases are part of, or regulate the activity of, intracellular molecular complexes, the inflammasomes, that sense "danger" to the body and coordinate an initial immune response. Our understanding of specific triggers of the inflammasomes, coupled with the recognition that inflammasomes are critical for activation of the proinflammatory cytokine IL-1, has provided a rational and very effective target in the treatment of a number of these rare autoinflammatory diseases. In addition, the ongoing discovery of the role of inflammasomes and IL-1 activation and secretion in a number of genetically complex disorders have fundamentally changed our view of disease pathogenesis in a growing number of disorders that were heretofore not even thought of as "immunologic" diseases.
Figures

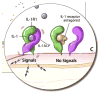
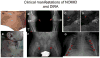
References
-
- The International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90:797–807. - PubMed
-
- The French FMF Consortium. A candidate gene for familial Mediterranean fever. Nat Genet. 1997;17:25–31. - PubMed
-
- McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–44. - PubMed
-
- Drenth JP, Cuisset L, Grateau G, Vasseur C, van de Velde-Visser SD, de Jong JG, et al. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat Genet. 1999;22:178–81. - PubMed
-
- Houten SM, Kuis W, Duran M, de Koning TJ, Royen-Kerkhof A, Romeijn GJ, et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobu-linaemia D and periodic fever syndrome. Nat Genet. 1999;22:175–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

