Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry
- PMID: 20004926
- PMCID: PMC3351100
- DOI: 10.1016/j.virol.2009.11.011
Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry
Abstract
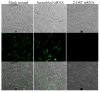
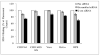
Heparan sulfate (HS) proteoglycans are commonly exploited by multiple viruses for initial attachment to host cells. Herpes simplex virus-1 (HSV-1) is unique because it can use HS for both attachment and penetration, provided specific binding sites for HSV-1 envelope glycoprotein gD are present. The interaction with gD is mediated by specific HS moieties or 3-O sulfated HS (3-OS HS), which are generated by all but one of the seven isoforms of 3-O sulfotransferases (3-OSTs). Here we demonstrate that several common experimental cell lines express unique sets of 3-OST isoforms. While the isoforms 3-OST-3, -5 and -6 were most commonly expressed, isoforms 3-OST-2 and -4 were undetectable in the cell lines examined. Since most cell lines expressed multiple 3-OST isoforms, we addressed the significance of 3-OS HS in HSV-1 entry by down-regulating 2-O-sulfation, a prerequisite for 3-OS HS formation, by knocking down 2-OST expression by RNA interference (RNAi). 2-OST knockdown was verified by reverse-transcriptase PCR and Western blot analysis, while 3-OS HS knockdown was verified by immunofluorescence. Cells showed a significant decrease in viral entry, suggesting an important role for 3-OS HS. Implicating 3-OS HS further, cells knocked down for 2-OST expression also demonstrated decreased cell-cell fusion when cocultivated with effector cells transfected with HSV-1 glycoproteins. Our findings suggest that 3-OS HS may play an important role in HSV-1 entry into many different cell lines.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Comprehensive analysis of herpes simplex virus 1 (HSV-1) entry mediated by zebrafish 3-O-Sulfotransferase isoforms: implications for the development of a zebrafish model of HSV-1 infection.J Virol. 2014 Nov;88(21):12915-22. doi: 10.1128/JVI.02071-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142596 Free PMC article.
-
Zebrafish 3-O-sulfotransferase-4 generated heparan sulfate mediates HSV-1 entry and spread.PLoS One. 2014 Feb 3;9(2):e87302. doi: 10.1371/journal.pone.0087302. eCollection 2014. PLoS One. 2014. PMID: 24498308 Free PMC article.
-
The principal neuronal gD-type 3-O-sulfotransferases and their products in central and peripheral nervous system tissues.Matrix Biol. 2007 Jul;26(6):442-55. doi: 10.1016/j.matbio.2007.03.002. Epub 2007 Mar 30. Matrix Biol. 2007. PMID: 17482450 Free PMC article.
-
Diversity of heparan sulfate and HSV entry: basic understanding and treatment strategies.Molecules. 2015 Feb 5;20(2):2707-27. doi: 10.3390/molecules20022707. Molecules. 2015. PMID: 25665065 Free PMC article. Review.
-
Herpes simplex virus: receptors and ligands for cell entry.Cell Microbiol. 2004 May;6(5):401-10. doi: 10.1111/j.1462-5822.2004.00389.x. Cell Microbiol. 2004. PMID: 15056211 Review.
Cited by
-
The effect of cellular differentiation on HSV-1 infection of oligodendrocytic cells.PLoS One. 2014 Feb 13;9(2):e89141. doi: 10.1371/journal.pone.0089141. eCollection 2014. PLoS One. 2014. PMID: 24551233 Free PMC article.
-
Overexpression of herpes simplex virus glycoprotein K (gK) alters expression of HSV receptors in ocularly-infected mice.Invest Ophthalmol Vis Sci. 2014 Apr 15;55(4):2442-51. doi: 10.1167/iovs.14-14013. Invest Ophthalmol Vis Sci. 2014. PMID: 24667863 Free PMC article.
-
Herpes simplex virus infects most cell types in vitro: clues to its success.Virol J. 2011 Oct 26;8:481. doi: 10.1186/1743-422X-8-481. Virol J. 2011. PMID: 22029482 Free PMC article. Review.
-
Specific sides to multifaceted glycosaminoglycans are observed in embryonic development.Semin Cell Dev Biol. 2010 Aug;21(6):631-7. doi: 10.1016/j.semcdb.2010.06.002. Epub 2010 Jul 3. Semin Cell Dev Biol. 2010. PMID: 20599516 Free PMC article. Review.
-
Effect of black tea extract on herpes simplex virus-1 infection of cultured cells.BMC Complement Altern Med. 2013 Jun 18;13:139. doi: 10.1186/1472-6882-13-139. BMC Complement Altern Med. 2013. PMID: 23777309 Free PMC article.
References
-
- Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol. 2000;10:305–319. - PubMed
-
- Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Sama D, Calatroni A. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes. J Cell Biochem. 2009;106:83–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

