Quantitative expression of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 in melanoma tissue microarrays
- PMID: 20004943
- PMCID: PMC2824079
- DOI: 10.1016/j.humpath.2009.08.016
Quantitative expression of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 in melanoma tissue microarrays
Abstract
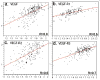
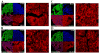
Angiogenesis is required for progression and metastasis of melanoma. Analysis of angiogenic molecules in benign and malignant tissues may allow identification of markers useful for prediction of sensitivity to antiangiogenic agents. We hypothesized that differential expression of vascular endothelial growth factor (VEGF) and its receptors VEGF-R1, VEGF-R2, and VEGF-R3 would be higher in melanomas than nevi and higher in advanced melanoma. Using automated quantitative analysis, we quantified VEGF, -R1, -R2 and -R3 expression in melanoma tissue microarrays composed of 540 nevi and 468 melanoma specimens (198 primaries, 270 metastases). VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 expression was significantly higher in melanomas than nevi by unpaired t tests (P < .0001). VEGF-R2 expression was higher in metastatic specimens (P < .0001), but VEGF-R3 expression was higher in primaries (P < .0001). VEGF was coexpressed with all 3 receptors when assessed by Spearman's rank correlation. VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 expression is higher in melanomas than nevi. Higher expression of VEGF-R2 was found in metastases versus primaries, supporting the idea that selection for an angiogenic phenotype in metastatic melanoma is conferred via up-regulation of VEGF-R2. However, higher expression of VEGF-R3 was seen on primary lesions, potentially implicating this receptor in initiation of lymphatic tumor spread. Clinical trials using antiangiogenic agents in melanoma should include correlative assays of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 as biomarkers of response to therapy, preferably using quantitative methods such as automated quantitative analysis. Such assessments could assist with evaluation of these molecules as therapeutic targets in melanoma, ultimately facilitating improved selection of patients for treatment.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. - PubMed
-
- Escudier B, Koralewski P, Pluzanska A, et al. A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-a2a vs placebo/interferon- a2a as first-line therapy in metastatic renal cell carcinoma. J Clin Oncol. 2007;25:2s. Abstract.
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. - PubMed
-
- Einspahr JG, Thomas TL, Saboda K, Nickolof BJ, Warneke J, Curiel-Lewandrowski C, Ranger-Moore J, Duckett L, Bangert J, Fruehauf JP, Alberts DS. Expression of vascular endothelial growth factor in early cutaneous melanocytic lesion progression. Cancer. 2007 Dec 1;110(11):2519–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

