Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus
- PMID: 20004959
- PMCID: PMC2989405
- DOI: 10.1016/j.cell.2009.11.034
Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus
Abstract
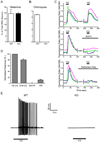
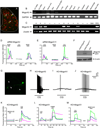
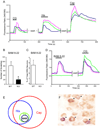
The cellular and molecular mechanisms mediating histamine-independent itch in primary sensory neurons are largely unknown. Itch induced by chloroquine (CQ) is a common side effect of this widely used antimalarial drug. Here, we show that Mrgprs, a family of G protein-coupled receptors expressed exclusively in peripheral sensory neurons, function as itch receptors. Mice lacking a cluster of Mrgpr genes display significant deficits in itch induced by CQ but not histamine. CQ directly excites sensory neurons in an Mrgpr-dependent manner. CQ specifically activates mouse MrgprA3 and human MrgprX1. Loss- and gain-of-function studies demonstrate that MrgprA3 is required for CQ responsiveness in mice. Furthermore, MrgprA3-expressing neurons respond to histamine and coexpress gastrin-releasing peptide, a peptide involved in itch sensation, and MrgprC11. Activation of these neurons with the MrgprC11-specific agonist BAM8-22 induces itch in wild-type but not mutant mice. Therefore, Mrgprs may provide molecular access to itch-selective neurons and constitute novel targets for itch therapeutics.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures


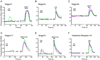

References
-
- Abila B, Ezeamuzie IC, Igbigbi PS, Ambakederemo AW, Asomugha L. Effects of two antihistamines on chloroquine and histamine induced weal and flare in healthy African volunteers. Afr J Med Med Sci. 1994;23:139–142. - PubMed
-
- Adam ME, Karim EF, Elkadaru AY, Ibrahim KE, Berger BJ, Wiese M, Babiker HA. Imipramine induced complete reversal of chloroquine resistance in plasmodium falciparum infections in Sudan. Saudi Pharmaceutical Journal. 2004;12:130–135.
-
- Ademowo OG, Walker O, Sodeinde O. Prevalence of chloroquine-induced pruritus: evidence for heredofamilial factors. Malaria Infect Dis Afr. 1998;8:14–18.
-
- Ajayi AA, Oluokun A, Sofowora O, Akinleye A, Ajayi AT. Epidemiology of antimalarial-induced pruritus in Africans. Eur J Clin Pharmacol. 1989;37:539–540. - PubMed
-
- Alving K, Sundstrom C, Matran R, Panula P, Hokfelt T, Lundberg JM. Association between histamine-containing mast cells and sensory nerves in the skin and airways of control and capsaicin-treated pigs. Cell Tissue Res. 1991;264:529–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- HL038095/HL/NHLBI NIH HHS/United States
- T32 EY017203/EY/NEI NIH HHS/United States
- P01 NS048499/NS/NINDS NIH HHS/United States
- NS048499/NS/NINDS NIH HHS/United States
- R01 AR056318/AR/NIAMS NIH HHS/United States
- R01 HL038095/HL/NHLBI NIH HHS/United States
- DK074480/DK/NIDDK NIH HHS/United States
- R01 DK074480/DK/NIDDK NIH HHS/United States
- R37 NS054791/NS/NINDS NIH HHS/United States
- AR056318/AR/NIAMS NIH HHS/United States
- NS054791/NS/NINDS NIH HHS/United States
- R01 NS054791/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

