A nonprocessive class V myosin drives cargo processively when a kinesin- related protein is a passenger
- PMID: 20005107
- PMCID: PMC2904613
- DOI: 10.1016/j.cub.2009.10.069
A nonprocessive class V myosin drives cargo processively when a kinesin- related protein is a passenger
Abstract
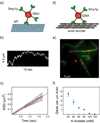
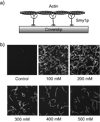
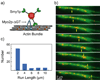
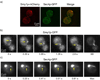
During secretory events, kinesin transports cargo along microtubules and then shifts control to myosin V for delivery on actin filaments to the cell membrane [1]. When kinesin and myosin V are present on the same cargo, kinesin interacts electrostatically with actin to enhance myosin V-based transport in vitro [2]. The relevance of this observation within the cell was questioned. In budding yeast, overexpression of a kinesin-family protein (Smy1p) suppressed a transport defect in a strain with a mutant class V myosin (Myo2p) [3]. We postulate that this is a cellular manifestation of the in vitro observation. We demonstrate that Smy1p binds electrostatically to actin bundles. Although a single Myo2p cannot transport cargo along actin bundles, addition of Smy1p causes the complex to undergo long-range, continuous movement. We propose that the kinesin-family protein acts as a tether that prevents cargo dissociation from actin, allowing the myosin to take many steps before dissociating. We demonstrate that both the tether and the motor reside on moving secretory vesicles in yeast cells, a necessary feature for this mechanism to apply in vivo. The presence of both kinesin and myosin on the same cargo may be a general mechanism to enhance cellular transport in yeast and higher organisms.
Figures
Similar articles
-
Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae.J Cell Biol. 1994 May;125(4):825-42. doi: 10.1083/jcb.125.4.825. J Cell Biol. 1994. PMID: 8188749 Free PMC article.
-
The yeast kinesin-related protein Smy1p exerts its effects on the class V myosin Myo2p via a physical interaction.Mol Biol Cell. 2000 Feb;11(2):691-702. doi: 10.1091/mbc.11.2.691. Mol Biol Cell. 2000. PMID: 10679024 Free PMC article.
-
Polarized distribution of intracellular components by class V myosins in Saccharomyces cerevisiae.Int Rev Cytol. 2003;229:1-42. doi: 10.1016/s0074-7696(03)29001-x. Int Rev Cytol. 2003. PMID: 14669953 Review.
-
Tropomyosin is essential for processive movement of a class V myosin from budding yeast.Curr Biol. 2012 Aug 7;22(15):1410-6. doi: 10.1016/j.cub.2012.05.035. Epub 2012 Jun 14. Curr Biol. 2012. PMID: 22704989 Free PMC article.
-
Walking to work: roles for class V myosins as cargo transporters.Nat Rev Mol Cell Biol. 2011 Dec 7;13(1):13-26. doi: 10.1038/nrm3248. Nat Rev Mol Cell Biol. 2011. PMID: 22146746 Review.
Cited by
-
Myosin Va participates in acrosomal formation and nuclear morphogenesis during spermatogenesis of Chinese mitten crab Eriocheir sinensis.PLoS One. 2010 Sep 14;5(9):e12738. doi: 10.1371/journal.pone.0012738. PLoS One. 2010. PMID: 20856877 Free PMC article.
-
Cytokinesis in Trypanosoma brucei relies on an orphan kinesin that dynamically crosslinks microtubules.Curr Biol. 2023 Mar 13;33(5):899-911.e5. doi: 10.1016/j.cub.2023.01.035. Epub 2023 Feb 13. Curr Biol. 2023. PMID: 36787745 Free PMC article.
-
The novel proteins Rng8 and Rng9 regulate the myosin-V Myo51 during fission yeast cytokinesis.J Cell Biol. 2014 May 12;205(3):357-75. doi: 10.1083/jcb.201308146. Epub 2014 May 5. J Cell Biol. 2014. PMID: 24798735 Free PMC article.
-
Transport of fungal RAB11 secretory vesicles involves myosin-5, dynein/dynactin/p25, and kinesin-1 and is independent of kinesin-3.Mol Biol Cell. 2017 Apr 1;28(7):947-961. doi: 10.1091/mbc.E16-08-0566. Epub 2017 Feb 16. Mol Biol Cell. 2017. PMID: 28209731 Free PMC article.
-
Bil2 Is a Novel Inhibitor of the Yeast Formin Bnr1 Required for Proper Actin Cable Organization and Polarized Secretion.Front Cell Dev Biol. 2021 Feb 9;9:634587. doi: 10.3389/fcell.2021.634587. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33634134 Free PMC article.
References
-
- Lillie SH, Brown SS. Suppression of a myosin defect by a kinesin-related gene. Nature. 1992;356:358–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

